M2780
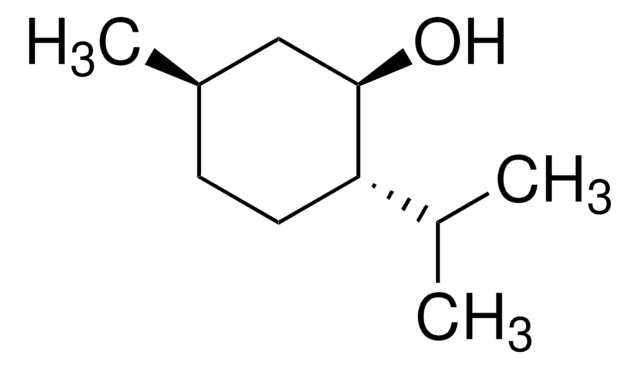
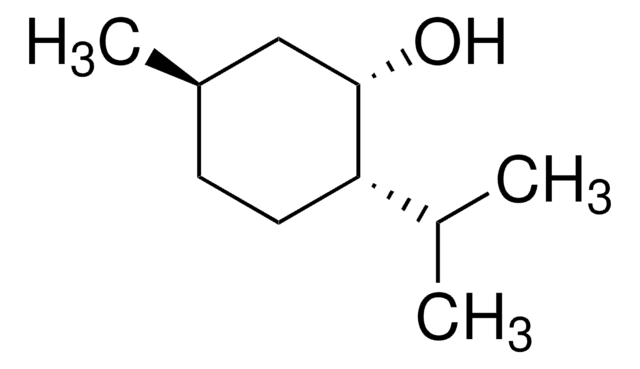
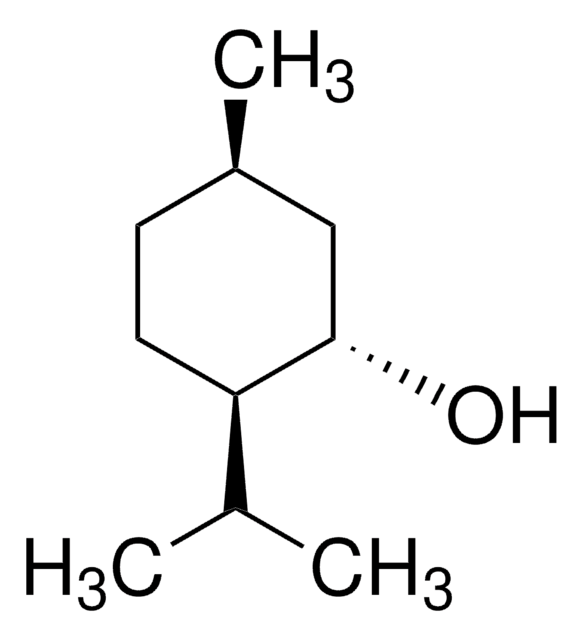
(1R,2S,5R)-(−)-Menthol
ReagentPlus®, 99%
Synonym(s):
(−)-Menthol, (1R,2S,5R)-2-Isopropyl-5-methylcyclohexanol, 5-Methyl-2-(1-methylethyl)cyclohexanol
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(3)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C10H20O
CAS Number:
Molecular Weight:
156.27
Beilstein:
1902293
EC Number:
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
biological source
synthetic (organic)
Quality Level
vapor pressure
0.8 mmHg ( 20 °C)
product line
ReagentPlus®
Assay
99%
optical activity
[α]20/D −50°, c = 10 in 95% ethanol
optical purity
ee: 99% (GLC)
bp
212 °C (lit.)
mp
41-45 °C (lit.)
solubility
water: 20 mg/mL, clear, colorless
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(1R,2S,5R)-(-)-Menthol is a monoterpene.
Application
(1R,2S,5R)-(-)-Menthol may be used to synthesize:
- chiral enantiopure 2-(1-hydroxyalkyl)pyridines, which can react with to form C3-symmetric tripodal ligands
- menthylphosphorodichioridite, a chiral derivatizing agent for the determination of enantiomeric purity of chiral diols or diamines by 31P NMR spectroscopy
- (2R,4S)-2,3,4,5-tetrahydro-2-(-)-menthyloxy-2-methyl-4-phenylpyrano-[3,2-c]-benzopyran-5-one, an intermediate to prepare a warfarin analog
Used to prepare reagents for chiral vinylogous Darzens and Reformatsky reactions. Important chiral auxiliary employed in the resolution of acids and for stereocontrolled synthesis.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
201.2 °F - closed cup
Flash Point(C)
94 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A new 31P NMR method for the enantiomeric excess determination of diols and secondary diamines with C 2 symmetry.
Brunel JM and Faure B.
Tetrahedron Asymmetry, 6(9), 2353-2356 (1995)
The Journal of Organic Chemistry, 52, 4397-4397 (1987)
Fieser, M.
Reagents for Organic Synthesis, 16, 203-203 (1992)
Marei GI, et al.
Pesticide Biochemistry and Physiology, 103(1), 56-61 (2012)
Oxindole alkaloids. A novel non-biomimetic entry to (-)-Horsfiline.
Palmisano G, et al.
Tetrahedron Asymmetry, 7(1), 1-4 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service