10850
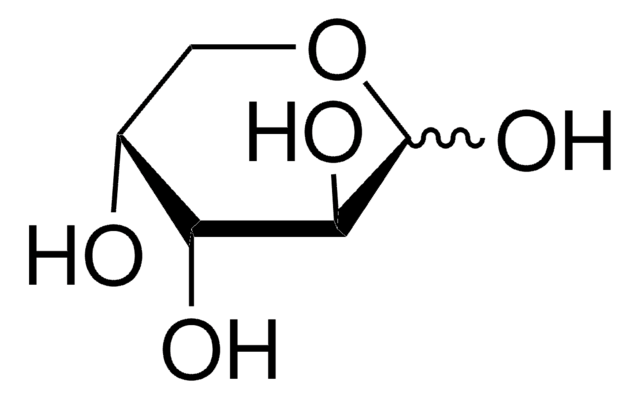
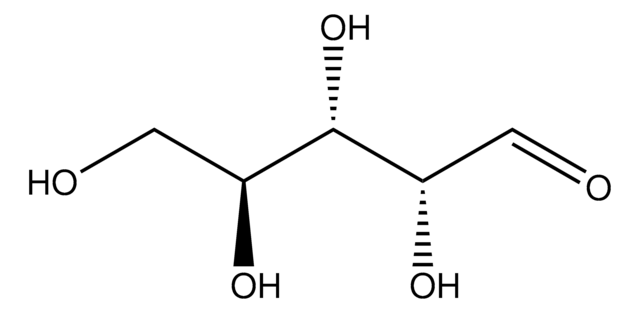
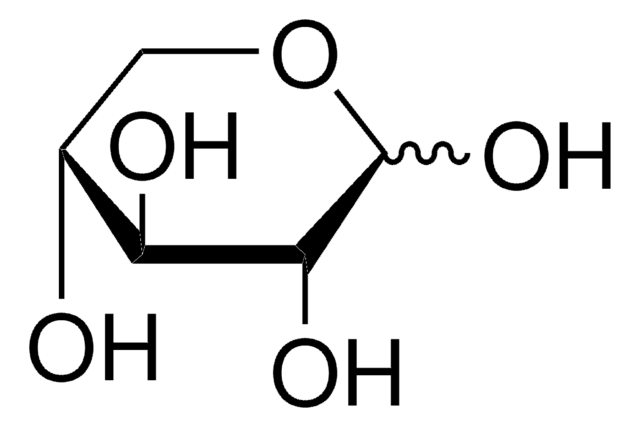
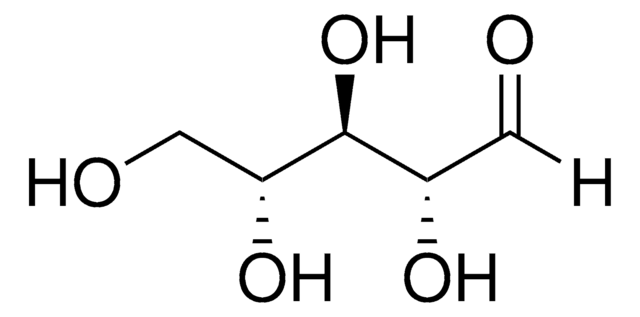
D-(−)-Arabinose
suitable for microbiology, ≥99.0%
About This Item
Recommended Products
Quality Level
Assay
≥99.0% (sum of enantiomers, HPLC)
≥99.0%
form
powder
optical activity
[α]20/D −104±2.0°, 24 hr, c = 10% in H2O
ign. residue
≤0.1% (as SO4)
loss
≤0.1% loss on drying, 20 °C (HV)
mp
162-164 °C (lit.)
anion traces
chloride (Cl-): ≤50 mg/kg
sulfate (SO42-): ≤50 mg/kg
cation traces
As: ≤0.1 mg/kg
Ca: ≤500 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤25 mg/kg
Fe: ≤17 mg/kg
K: ≤50 mg/kg
Mg: ≤10 mg/kg
Mn: ≤5 mg/kg
Na: ≤50 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤15 mg/kg
application(s)
microbiology
Looking for similar products? Visit Product Comparison Guide
Related Categories
1 of 4
This Item | A3131 | PHR2100 | 10839 |
|---|---|---|---|
| Quality Level 200 | Quality Level 200 | Quality Level 300 | Quality Level 100 |
| form powder | form powder | form - | form powder |
| mp 162-164 °C (lit.) | mp 162-164 °C (lit.) | mp 162-164 °C (lit.) | mp 155-159 °C, 160-163 °C (lit.) |
| cation traces As: ≤0.1 mg/kg, Cd: ≤5 mg/kg, Cr: ≤5 mg/kg, Fe: ≤17 mg/kg, Mg: ≤10 mg/kg, Na: ≤50 mg/kg, Pb: ≤5 mg/kg | cation traces - | cation traces - | cation traces Al: ≤5 mg/kg, As: ≤0.1 mg/kg, Ba: ≤5 mg/kg, Bi: ≤5 mg/kg, Ca: ≤200 mg/kg, Cd: ≤5 mg/kg, Co: ≤5 mg/kg, Cr: ≤5 mg/kg, Cu: ≤5 mg/kg, Fe: ≤5 mg/kg, K: ≤50 mg/kg, Li: ≤5 mg/kg, Mg: ≤5 mg/kg, Mn: ≤5 mg/kg, Mo: ≤5 mg/kg, Na: ≤50 mg/kg, Ni: ≤5 mg/kg, Pb: ≤5 mg/kg, Sr: ≤5 mg/kg, Zn: ≤5 mg/kg |
| optical activity [α]20/D −104±2.0°, 24 hr, c = 10% in H2O | optical activity [α]20/D -105 to -103 °, c = 4% (w/v) in water | optical activity - | optical activity [α]20/D +104.0±2.0°, 24 hr, c = 10% in H2O |
General description
Application
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service