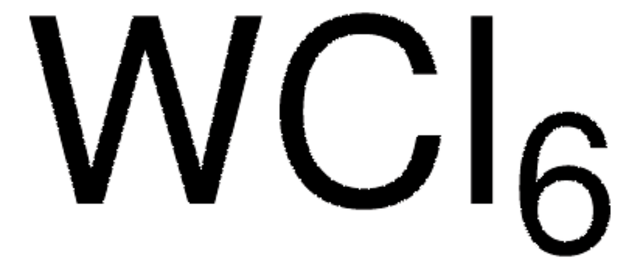590592
Hafnium(IV) chloride
purified by sublimation, 99.9% trace metals basis
Synonym(s):
Hafnium tetrachloride, Tetrachlorohafnium
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HfCl4
CAS Number:
Molecular Weight:
320.30
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
grade:
for analytical purposes
form:
powder
Recommended Products
grade
for analytical purposes
vapor pressure
1 mmHg ( 190 °C)
Assay
99.9% trace metals basis
form
powder
purified by
sublimation
impurities
≤1500.0 ppm Trace Metal Analysis
SMILES string
Cl[Hf](Cl)(Cl)Cl
InChI
1S/4ClH.Hf/h4*1H;/q;;;;+4/p-4
InChI key
PDPJQWYGJJBYLF-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Application
Hafnium(IV) chloride can be used as a catalyst:
- To synthesize α-aminophosphonates via the one-pot three-component reaction of aldehyde, amine, and phosphite.
- For the conversion of carbohydrates into 5-hydroxymethylfurfural in ionic liquids.
- For parallel synthesis of 1,2-disubstituted benzimidazoles from N-substituted phenylenediamines and aldehydes.
- A precursor to prepare hafnium oxide nanoparticles via sol-gel method.
- A starting material to synthesize bulky guanidinato hafnium(iv) chloridecomplexes.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Visualization of the cytoskeletal elements in tissue culture cells by bloc-staining with hafnium chloride after rapid freezing and freeze-substitution fixation.
T Hatae et al.
Journal of electron microscopy, 33(2), 186-190 (1984-01-01)
Guoguang Liu et al.
Biomacromolecules, 9(3), 949-953 (2008-02-15)
The feasibility of a previously established method based on ozonolysis and hydrogenation reactions for the production of 9-hydroxynonanoic acid from oleic acid has been demonstrated. Metal catalyzed lactonization conditions have been used to convert 9-hydroxynonanoic acid into 1,11-dioxacycloicosane-2,12-dione, which is
Lutz Ackermann et al.
Organic & biomolecular chemistry, 5(12), 1975-1978 (2007-06-07)
Two distinct economical catalysts for intramolecular hydroaminations of electronically unactivated alkenes with basic amines are described, which are based on (a) group 4 metal halides under basic reaction conditions or (b) Brønsted-acid organocatalysts.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service