329118
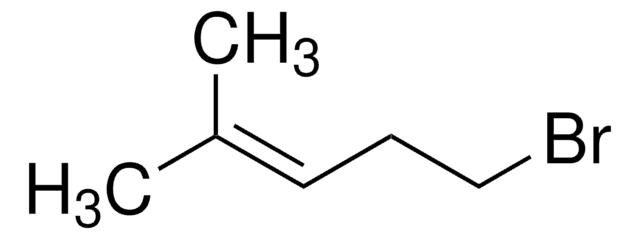
Geranyl bromide
95%
Synonym(s):
trans-1-Bromo-3,7-dimethyl-2,6-octadiene
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
(CH3)2C=CHCH2CH2C(CH3)=CHCH2Br
CAS Number:
Molecular Weight:
217.15
Beilstein:
1703631
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.504 (lit.)
bp
101-102 °C/12 mmHg (lit.)
density
1.094 g/mL at 25 °C (lit.)
functional group
alkyl halide
bromo
storage temp.
2-8°C
SMILES string
C\C(C)=C\CC\C(C)=C\CBr
InChI
1S/C10H17Br/c1-9(2)5-4-6-10(3)7-8-11/h5,7H,4,6,8H2,1-3H3/b10-7+
Looking for similar products? Visit Product Comparison Guide
General description
Geranyl bromide undergoes palladium catalyzed cross-coupling reaction with aryl and alkenylgold(I) phosphanes.
Application
Geranyl bromide was used in synthesis of baicalein and 3,7-dihydroxyflavone derivatives. It was also used in synthesis of potential flavonoidic modulators of P-glycoprotein activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
167.0 °F - closed cup
Flash Point(C)
75 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Maitrejean et al.
Bioorganic & medicinal chemistry letters, 10(2), 157-160 (2000-02-15)
A new series of potential flavonoidic modulators of P-glycoprotein activity has been prepared. The flavanolignan silybin was first oxidised to dehydrosilybin and then C-alkylated with either prenyl or geranyl bromide. The resulting isoprenoid dehydrosilybins were shown to display high in
Marta Perro Neves et al.
European journal of medicinal chemistry, 46(6), 2562-2574 (2011-04-19)
Fourteen baicalein and 3,7-dihydroxyflavone derivatives were synthesized and evaluated for their inhibitory activity against the in vitro growth of three human tumor cell lines. The synthetic approaches were based on the reaction with prenyl or geranyl bromide in alkaline medium
Palladium-catalyzed cross-coupling reactions of organogold(I) phosphanes with allylic electrophiles.
Miguel Peña-López et al.
Organic & biomolecular chemistry, 10(8), 1686-1694 (2012-01-24)
Aryl and alkenylgold(I) phosphanes react regioselectively with allylic electrophiles such as cinnamyl and geranyl halides (bromide, chloride and acetates) under palladium catalysis in THF at 80 °C to afford the α-substitution product with moderate to high yields. When the reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service