252786
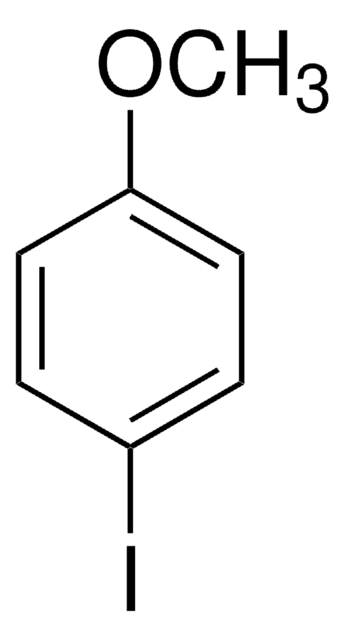
2-Iodoanisole
98%
Synonym(s):
2-Iodophenyl methyl ether, 2-Methoxyiodobenzene
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
IC6H4OCH3
CAS Number:
Molecular Weight:
234.03
Beilstein:
1860243
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.622 (lit.)
bp
125-126 °C/19 mmHg (lit.)
solubility
alcohol: miscible(lit.)
chloroform: miscible(lit.)
diethyl ether: miscible(lit.)
water: insoluble(lit.)
density
1.799 g/mL at 25 °C (lit.)
functional group
iodo
SMILES string
COc1ccccc1I
InChI
1S/C7H7IO/c1-9-7-5-3-2-4-6(7)8/h2-5H,1H3
Looking for similar products? Visit Product Comparison Guide
General description
2-Iodoanisole participates in palladium catalyzed enantioselective Heck arylation of 2,3-dihydrofuran in the presence of chiral ionic liquids containing L-prolinate and L-lactate anions and non-chiral quaternary ammonium cations.
Application
2-Iodoanisole has been used in palladium/copper-catalyzed synthesis of o-(1-alkynyl)anisoles.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adam Morel et al.
Dalton transactions (Cambridge, England : 2003), 42(4), 1215-1222 (2012-11-09)
Chiral ionic liquids (CILs) containing L-prolinate and L-lactate anions and non-chiral quaternary ammonium cations were employed in the palladium catalyzed enantioselective Heck arylation of 2,3-dihydrofuran with aryl iodides (iodobenzene, 4-iodotoluene, 2-iodoanisole, 4-iodoanisole, 4-iodoacetophenone). In all the reactions 2-aryl-2,3-dihydrofuran (3) was
Dawei Yue et al.
The Journal of organic chemistry, 70(25), 10292-10296 (2005-12-06)
[reaction: see text] 2,3-Disubstituted benzo[b]furans are readily prepared under very mild reaction conditions by the palladium/copper-catalyzed cross-coupling of various o-iodoanisoles and terminal alkynes, followed by electrophilic cyclization with I2, PhSeCl, or p-O2NC6H4SCl. Aryl- and vinylic-substituted alkynes undergo electrophilic cyclization in
Naoyuki Yasaka et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 34(10), 1183-1188 (2018-10-12)
Aryl halides are a very important category of compounds that include many vital drugs and key industrial additives, such as clofibrate and bromobenzene, respectively. Due to their importance, our research group previously developed a novel fluorescence labeling approach for their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service