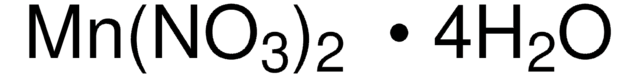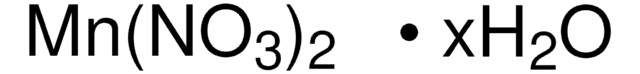203742
Manganese(II) nitrate hydrate
99.99% trace metals basis
Synonym(s):
Manganous dinitrate hydrate
Select a Size
Select a Size
About This Item
Recommended Products
Quality Level
Assay
99.99% trace metals basis
form
crystals and lumps
composition
Degree of hydration, 4-6
impurities
≤150.0 ppm Trace Metal Analysis
application(s)
battery manufacturing
storage temp.
2-8°C
SMILES string
O.[Mn++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Mn.2NO3.H2O/c;2*2-1(3)4;/h;;;1H2/q+2;2*-1;
InChI key
HBTFASPVVFSRRI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- To fabricate tetragonal LiMn2O4 as a dual-functional pseudocapacitor-battery electrode via an in-situ ion-exchange method.
- As a safe, cost-effective, and efficient electrolyte for rapid energy storage applications like supercapacitors.
- As a precursor to prepare hybrid catalyst to enhance contaminants poisoning tolerance in solid oxide fuel cell cathodes.
- To prepare MnO2–CoO catalyst to fabricate opto-electronic humidity sensors.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service