110388
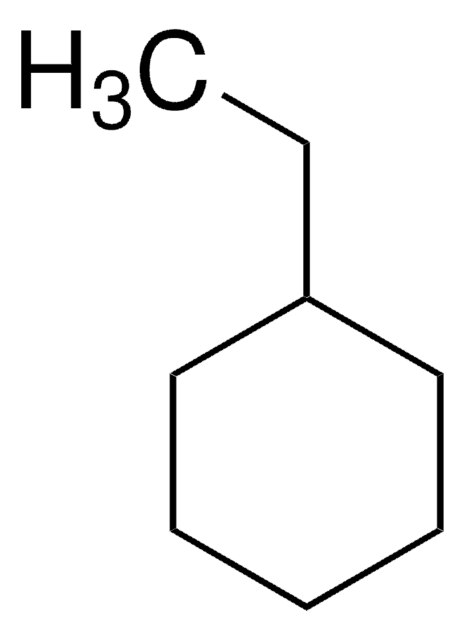
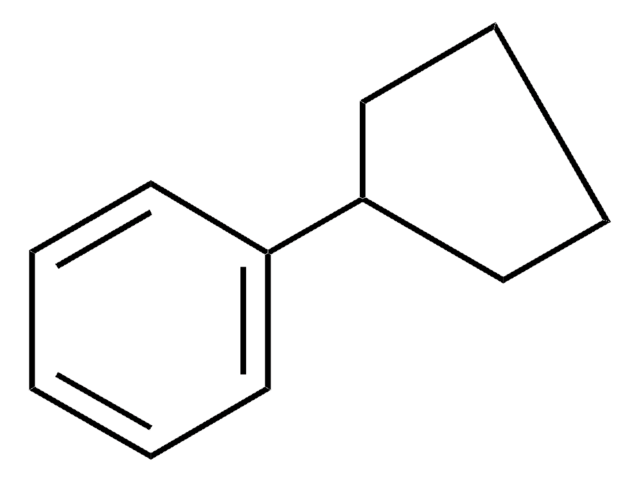
Butylcyclohexane
≥99%
Synonym(s):
1-Cyclohexylbutane
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
CH3(CH2)3C6H11
CAS Number:
Molecular Weight:
140.27
Beilstein:
1900631
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
2.9 mmHg ( 37.7 °C)
Quality Level
Assay
≥99%
autoignition temp.
475 °F
refractive index
n20/D 1.441 (lit.)
bp
178-180 °C (lit.)
mp
−78 °C (lit.)
density
0.818 g/mL at 25 °C (lit.)
SMILES string
CCCCC1CCCCC1
InChI
1S/C10H20/c1-2-3-7-10-8-5-4-6-9-10/h10H,2-9H2,1H3
Looking for similar products? Visit Product Comparison Guide
General description
Butylcyclohexane reacts with n-butylbenzene by free-radical mechanism to form 1-methylcyclohexene and cyclohexane. Butylcyclohexane, when photo-oxidized by solar light on synthetic seawater, forms cyclohexanone.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
105.8 °F - closed cup
Flash Point(C)
41 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thermal Decomposition of Jet Fuel Model Compounds under Near-Critical and Supercritical Conditions. 1. n-Butylbenzene and n-Butylcyclohexane.
Industrial & Engineering Chemistry Research, 37(12), 4591-4600 (1998)
Photosensitized oxidation of n-butylcyclohexane as a model for photochemical degradation of n-alkylcyclohexanes in seawater.
Journal of Photochemistry and Photobiology A: Chemistry, 46(3), 347-355 (1989)
Carmen M Dominguez et al.
Journal of environmental management, 261, 110240-110240 (2020-03-10)
Chlorinated pesticides were extensively produced in the XX century, generating high amounts of toxic wastes often dumped in the surroundings of the production sites, resulting in hot points of soil and groundwater pollution worldwide. This is the case of Bailín
Emre Seyyal et al.
Analytica chimica acta, 964, 96-111 (2017-03-30)
Principles of sol-gel chemistry were utilized to create silica- and germania-based dual-ligand surface-bonded sol-gel coatings providing enhanced performance in capillary microextraction (CME) through a combination of ligand superhydrophobicity and π-π interaction. These organic-inorganic hybrid coatings were prepared using sol-gel precursors
Jae-Man Park et al.
Nature communications, 11(1), 4638-4638 (2020-09-17)
Existing gels are mostly polar, whose nature limits their role in soft devices. The intermolecular interactions of nonpolar polymer-liquid system are typically weak, which makes the gel brittle. Here we report highly soft and transparent nonpolar organogels. Even though their
Protocols
-Xylene; Nonane; Decane; 1,2,4-Trimethylbenzene; Butylcyclohexane; Naphthalene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service