추천 제품
Grade
pharmaceutical primary standard
API family
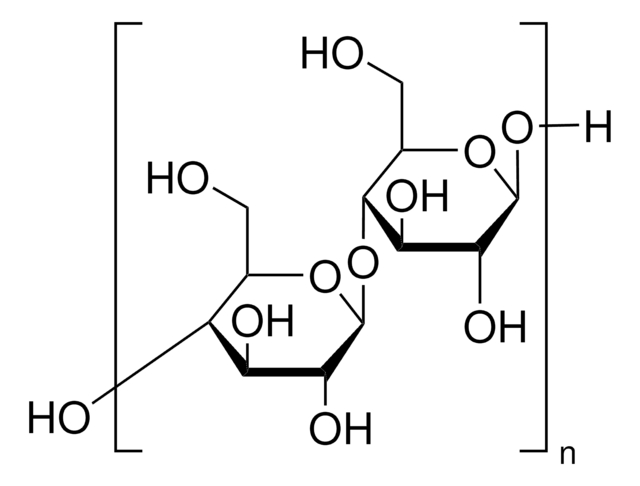
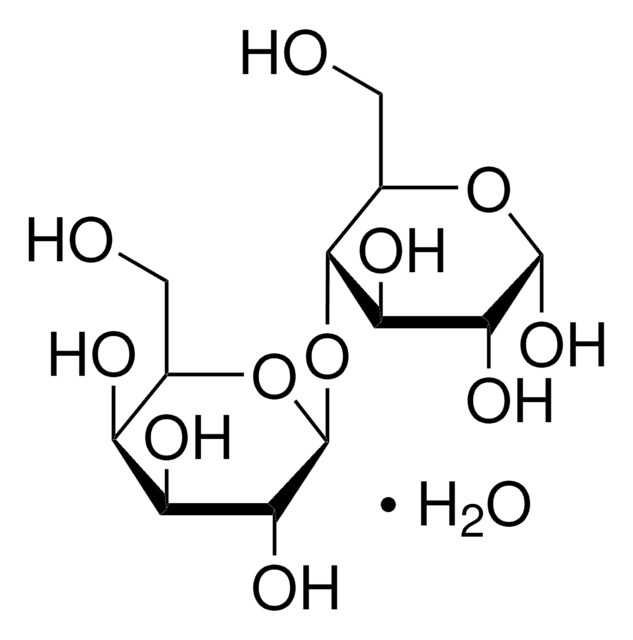
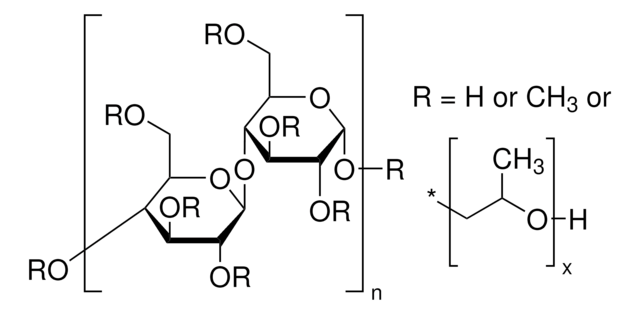
starch
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Sodium Starch Glycolate Type A USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monograph such as Sodium Starch Glycolate
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
M Maghsoodi et al.
Pharmaceutical development and technology, 16(3), 243-249 (2010-02-24)
Spherical crystallization (SC) of carbamazepine (CBZ) was carried out for preparation of the agglomerates using the solvent change method. The potential of the intraagglomerate addition of sodium starch glycolate (SSG) as a disintegrant agent and povidone (PVP) as a hydrophilic
Aiman A Obaidat et al.
Acta pharmaceutica (Zagreb, Croatia), 61(1), 83-91 (2011-03-17)
The aim of this study was to prepare fast-dissolving tablets of meloxicam after its complexation with β-cyclodextrin (β-CD) and to investigate the effect of using different superdisintegrants on the disintegration and release of meloxicam from the tablets. A complex of
Sagarika Bose et al.
International journal of pharmaceutics, 393(1-2), 32-40 (2010-02-09)
Film coating is generally accomplished by spraying polymers dissolved in solvents onto a cascading bed of tablets. The limitations associated with the use of solvents (both aqueous and organic) can be overcome by the use of solventless coating technologies. In
Balasubramaniam Jagadish et al.
Chemical & pharmaceutical bulletin, 58(3), 293-300 (2010-03-02)
The present study investigated the effect of co-grinding raloxifene HCL (RHCL) with different superdisintegrants, namely crospovidone (CP), croscarmellose sodium (CCS) and sodium starch glycolate (SSG), using a ball mill, in order to determine the potential effect on dissolution rate and
Ramji Anil Kumar Arza et al.
AAPS PharmSciTech, 10(1), 220-226 (2009-03-12)
Drugs that have narrow absorption window in the gastrointestinal tract (GIT) will have poor absorption. For these drugs, gastroretentive drug delivery systems offer the advantage in prolonging the gastric emptying time. Swellable, floating, and sustained release tablets are developed by
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.