1347700
USP
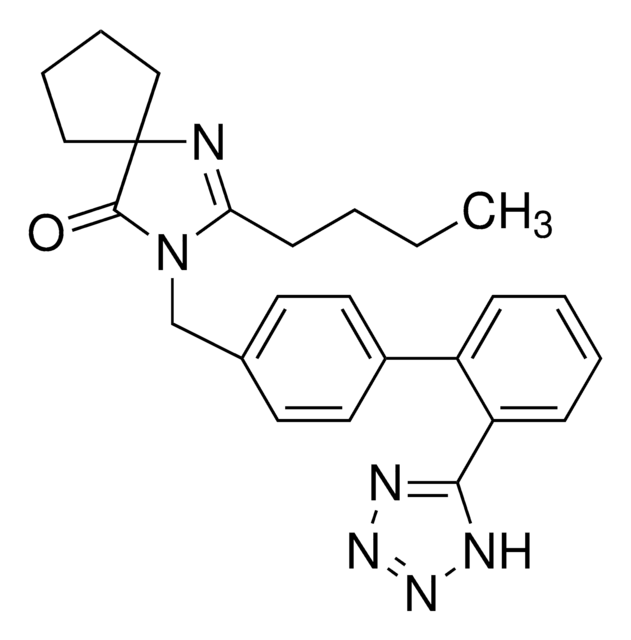
Irbesartan
United States Pharmacopeia (USP) Reference Standard
동의어(들):
2-Butyl-3-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C25H28N6O
CAS Number:
Molecular Weight:
428.53
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
irbesartan
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
CCCCC1=NC2(CCCC2)C(=O)N1Cc3ccc(cc3)-c4ccccc4-c5nnn[nH]5
InChI
1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30)
InChI key
YOSHYTLCDANDAN-UHFFFAOYSA-N
유전자 정보
human ... AGTR1(185)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Irbesartan USP Reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Irbesartan Tablets
- Irbesartan and Hydrochlorothiazide Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
J C Gillis et al.
Drugs, 54(6), 885-902 (1998-01-09)
Irbesartan inhibits the activity of angiotensin II (AII) via specific, selective noncompetitive antagonism of the AII receptor subtype 1 (AT1) which mediates most of the known physiological activities of AII. In patients with mild to moderate hypertension, once daily administration
Optimizing antiplatelet therapy in high-risk patients with atrial fibrillation: insights from Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE A).
Raymond Chee-Seong Seet et al.
Stroke, 40(12), 3883-3885 (2009-10-31)
Katherine F Croom et al.
Drugs, 68(11), 1543-1569 (2008-07-17)
Irbesartan (Aprovel, Avapro, Irbetan, Karvea), an angiotensin II receptor type 1 antagonist, is approved in many countries worldwide for the treatment of hypertension. It is also approved in some regions for the treatment of nephropathy in patients with hypertension and
Andrea Hartner et al.
Biochimica et biophysica acta, 1842(4), 558-565 (2014-01-15)
Diabetes can disrupt endoplasmic reticulum (ER) homeostasis which leads to ER stress. ER stress-induced renal apoptosis seems to be involved in the development of diabetic nephropathy. The present study was designed to investigate the contribution of reduced ER stress to
Karly P Garnock-Jones
American journal of cardiovascular drugs : drugs, devices, and other interventions, 13(2), 141-150 (2013-03-22)
Combination therapy is often required in patients with hypertension, and fixed-dose single-pill combinations have been shown to provide an easier regimen for patients, improving adherence. Irbesartan/amlodipine (Aprovasc®) is an angiotensin-receptor blocker/calcium-channel blocker fixed-dose single-pill combination, whose constituent drugs exert additive
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.