추천 제품
일반 설명
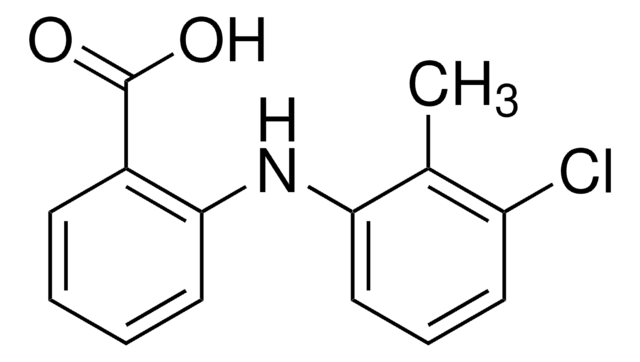
Tolfenamic acid is a fenamate and is made up of a monocarboxylate diphenylamine nucleus.
애플리케이션
Tolfenamic acid has been used in Arabidopsis bud assay for activity comparison with quinazolinedione derivatives (QADD) based compounds.
생화학적/생리학적 작용
Non-steroidal anti-inflammatory agent. Interferes with synthesis of β-amyloid precursor protein, and thus Aβ peptides, by promoting degradation of an essential transcription factor.
Tolfenamic acid is involved in the inhibition of prostaglandin synthesis. It also plays a role in apoptosis of head and neck cancer cells.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
R A Tokola et al.
Cephalalgia : an international journal of headache, 4(4), 253-263 (1984-12-01)
Tolfenamic acid is a fenamate which inhibits prostaglandin (PG) biosynthesis and may act as a PG antagonist as well. Caffeine and metoclopramide are used in combination with analgesics and ergotamine in the treatment of migraine attacks, but controlled clinical studies
Don Eslin et al.
Molecular carcinogenesis, 52(5), 377-386 (2012-01-04)
Current therapeutic options for recurrent neuroblastoma have poor outcomes that warrant the development of novel therapeutic strategies. Specificity protein (Sp) transcription factors regulate several genes involved in cell proliferation, survival, and angiogenesis. Sp1 regulates genes believed to be important determinants
Sung Un Kang et al.
PloS one, 7(4), e34988-e34988 (2012-04-27)
Nonsteroidal anti-inflammatory drug-activated gene-1 (NAG-1) is induced by nonsteroidal anti-inflammatory drugs and possesses proapoptotic and antitumorigenic activities. Although tolfenamic acid (TA) induces apoptosis in head and neck cancer cells, the relationship between NAG-1 and TA has not been determined. This
Alketa Tarushi et al.
Dalton transactions (Cambridge, England : 2003), 41(23), 7082-7091 (2012-05-05)
The interaction of Zn(II) with the non-steroidal anti-inflammatory drug tolfenamic acid leads to the formation of the structurally characterized trinuclear [Zn(3)(tolfenamato)(6)(CH(3)OH)(2)] complex. In the presence of the N,N'-donor heterocyclic ligands 1,10-phenanthroline and 2,2'-bipyridine at a range of ratios, the mononuclear
Alessandra Mattei et al.
Pharmaceutical research, 29(2), 460-470 (2011-09-01)
Crystallization from solution involves nucleation and growth; growth conditions greatly influence self-association behaviors of solute molecules in these steps, affecting crystal packing of organic molecules. We examined the role of pre-nucleation association to provide insights into the mutual influence between
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.