추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
저장 조건
desiccated
색상
white to brown
solubility
DMSO: 10 mg/mL, clear
저장 온도
−20°C
SMILES string
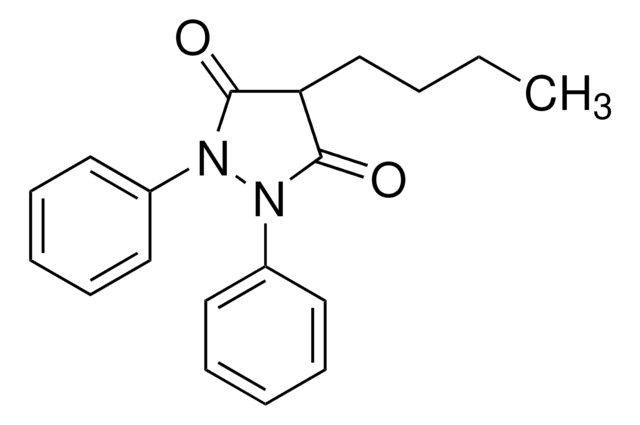
N2(N(C(=O)C(C2=O)CCCC)c3ccccc3)c1ccc(cc1)O
InChI
1S/C19H20N2O3/c1-2-3-9-17-18(23)20(14-7-5-4-6-8-14)21(19(17)24)15-10-12-16(22)13-11-15/h4-8,10-13,17,22H,2-3,9H2,1H3
InChI key
HFHZKZSRXITVMK-UHFFFAOYSA-N
일반 설명
Oxyphenbutazone is a derivative compound of phenylbutazone.
생화학적/생리학적 작용
Oxyphenbutazone is a non-steroid anti inflammatory; anti Mycobacterium tuberculosis agent.
Oxyphenbutazone is a non-steroid anti inflammatory; anti Mycobacterium tuberculosis agent. Oxyphenbutazone is known to cause inflammatory effects on tissues. Oxyphenbutazone, as a drug, decreases cellular exudates, without involving the pituitary-adrenal axis or the immunity response. Though the drug delivers a number of side effects, it is considered to be less toxic than phenylbutazone, due to decreased rate of intestinal absorption.
Oxyphenbutazone is an NSAID that has been shown to preferentially kill non-replicating Mycobaterium tuberculosis maintained in media that simulates the mildly acidic, in vivo conditions where drug-resistant, non replicating subpopulations of the bacteria reside in hosts. The compound has little or no affect on replication M. tuberculosis grown in normal liquid cultures.
기타 정보
Air sensitive
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Aik-Jiang Lau et al.
Journal of pharmaceutical and biomedical analysis, 31(2), 401-406 (2003-03-01)
Adulterations with synthetic drugs are common problems with herbal medicine and this can potentially cause serious adverse effects. It is therefore important to determine the presence of synthetic drugs in herbal medicine to ensure patients' safety. The objective of this
Youwen You et al.
Journal of analytical toxicology, 33(1), 41-50 (2009-01-24)
A sensitive liquid chromatographic-tandem mass spectrometric method was developed and validated for screening, quantification, and confirmation of phenylbutazone and oxyphenbutazone in equine plasma. Analytes were recovered from plasma by liquid-liquid extraction followed by separation in a reversed-phase column and identification
E Pesce et al.
Research communications in molecular pathology and pharmacology, 115-116, 39-48 (2007-06-15)
The antirheumatic effect of pirfenidone was compared with a positive control drug, oxyphenbutazone which is used in patients suffering from rheumatoid arthritis, in a double blind clinical trial in humans. The data collected in this pilot project revealed that pirfenidone
N S Matthews et al.
American journal of veterinary research, 62(5), 673-675 (2001-05-09)
To describe the pharmacokinetics of phenylbutazone and oxyphenbutazone after IV administration in miniature donkeys. 6 clinically normal miniature donkeys. Blood samples were collected before and 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, 300, 360, and 480 minutes
Ulcer of the cecum during oxyphenbutazone (tandearil) therapy.
Debenham G P
Canadian Medical Association Journal, 94(22), 1182-1182 (1966)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.