추천 제품
생물학적 소스
bovine milk
Quality Level
분석
≥99% (TLC)
양식
powder or crystals
광학 활성
[α]20/D 173 to 180°, c = 1.5% (w/v) in water
기술
thin layer chromatography (TLC): suitable
색상
white
mp
116-117 °C (lit.)
solubility
water: 50 mg/mL, clear, colorless
저장 온도
2-8°C
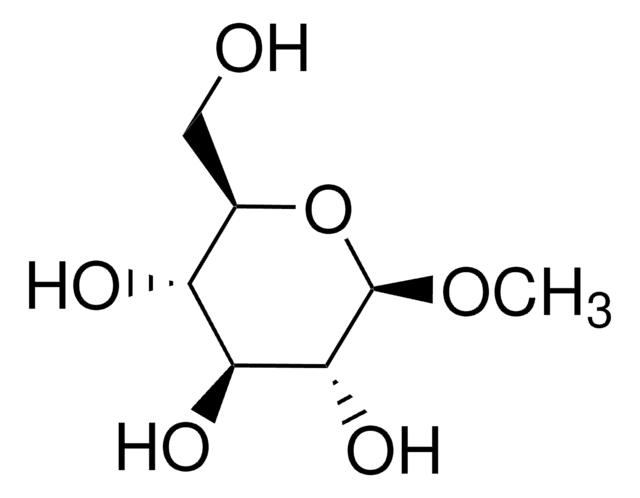
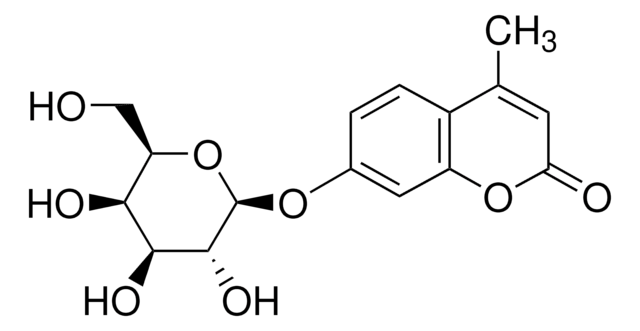
SMILES string
CO[C@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C7H14O6/c1-12-7-6(11)5(10)4(9)3(2-8)13-7/h3-11H,2H2,1H3/t3-,4+,5+,6-,7+/m1/s1
InChI key
HOVAGTYPODGVJG-PZRMXXKTSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Methyl α-D-galactopyranoside is potent inhibitor against the Debaryomyces hansenii UFV-1 extracellular and intracellular α-galactosidases.
애플리케이션
Methyl α-D-galactopyranoside has been used in computational studies of protonated β-d-galactose and its hydrated complex.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Hong-bin Xie et al.
The journal of physical chemistry. B, 116(16), 4851-4859 (2012-04-12)
We present an exploration of proton transfer dynamics in a monosaccharide, based upon ab initio molecular dynamic (AIMD) simulations, conducted "on-the-fly", in β-d-galactose-H(+) (βGal-H(+)) and its singly hydrated complex, βGal-H(+)-H2O. Prior structural calculations identify O6 as the preferred protonation site
Pollyanna A Viana et al.
Carbohydrate research, 346(5), 602-605 (2011-02-25)
α-D-Galactopyranosides were synthesized and their inhibitory activities toward the Debaryomyces hansenii UFV-1 extracellular and intracellular α-galactosidases were evaluated. Methyl α-D-galactopyranoside was the most potent inhibitor compared to the others tested, with K(i)(') values of 0.82 and 1.12 mmolL(-1), for extracellular
Feng Yang et al.
Carbohydrate research, 337(6), 485-491 (2002-03-14)
Oligosaccharide derivatives from sanqi, a Chinese herbal medicine derived from Panax notoginseng, methyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, diosgenyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, and methyl beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranosyl-(1-->4)-beta-D-galactopyranosyl-(1-->3)-[alpha-L-arabinofuranosyl-(1-->6)]-alpha-D-galactopyranoside, were synthesized under standard glycosylation conditions. An unexpected alpha-(1-->4) linkage was formed predominantly in the presence of neighboring participation group during
Joel S Griffitts et al.
Science (New York, N.Y.), 307(5711), 922-925 (2005-02-12)
The development of pest resistance threatens the effectiveness of Bacillus thuringiensis (Bt) toxins used in transgenic and organic farming. Here, we demonstrate that (i) the major mechanism for Bt toxin resistance in Caenorhabditis elegans entails a loss of glycolipid carbohydrates;
J W Dallinga et al.
Biological mass spectrometry, 23(12), 764-770 (1994-12-01)
The fast atom bombardment collision-induced dissociation mass spectra of the [M-H]- ions of the 2-, 3-, 4- and 6-deoxy derivatives from methyl beta-D-galactopyranoside and some related compounds have been recorded. The fragmentation reactions of these quasimolecular ions and of OD-labelled
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.