모든 사진(1)
About This Item
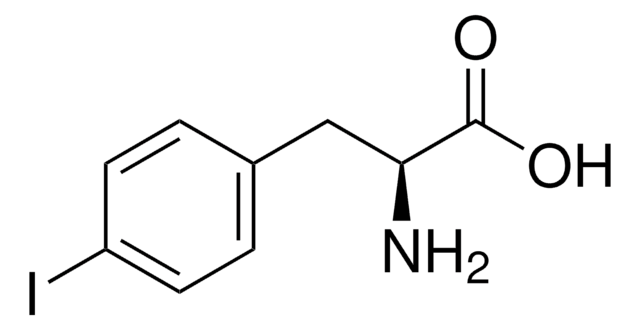
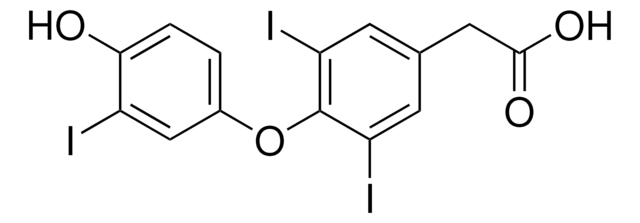
Linear Formula:
IC6H3-4-(OH)CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
307.09
Beilstein:
2941266
EC Number:
MDL number:
UNSPSC 코드:
12352200
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.32
추천 제품
Quality Level
불순물
~5% tyrosine
mp
210 °C (dec.) (lit.)
solubility
dilute aqueous acid: soluble
저장 온도
−20°C
SMILES string
N[C@@H](Cc1ccc(O)c(I)c1)C(O)=O
InChI
1S/C9H10INO3/c10-6-3-5(1-2-8(6)12)4-7(11)9(13)14/h1-3,7,12H,4,11H2,(H,13,14)/t7-/m0/s1
InChI key
UQTZMGFTRHFAAM-ZETCQYMHSA-N
유전자 정보
human ... TH(7054)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Iodotyrosine coupled with di-iodotyrosine results in the synthesis of 3,5,3′-tri-iodothyronine (T3) or 3,3′,5′-tri-iodothyronine (rT3).
애플리케이션
3-Iodo-L-tyrosine has been used as an inhibitor for tyrosine hydroxylase enzyme in Drosophila and silkworm pupae.
생화학적/생리학적 작용
3-iodotyrosine (3-IY) inhibits tyrosine hydroxylase that catalyzes levodopa (L-DOPA) formation from tyrosine. Iodotyrosine deiodinase enzyme deficiency leads to elevated levels of 3-IY in serum and urine in severe hypothyroidism and goiter.
TH (tyrosine 3-hydroxylase) is responsible for catalyzing the first step of the noradrenergic biosynthesis pathway. Mutations in TH are associated with tyrosine hydroxylase deficiency, leading to conditions such as infantile parkinsonism and DOPA (dopamine)-responsive dystonia as well as encephalopathy with perinatal onset.
Tyrosine hydroxylase inhibitor.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
L G Harsing et al.
Neuroscience, 77(2), 419-429 (1997-03-01)
Striatal slices from the rat were preincubated with [3H]GABA and superfused in the presence of nipecotic acid and aminooxyacetic acid, inhibitors of high-affinity GABA transport and GABA aminotransferase, respectively. GABA efflux was estimated by monitoring tritium efflux, 98% of which
D K Ness et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 30(2), 153-161 (1996-04-01)
Planarians (Dugesia dorotocephala) were evaluated as bioassay organisms to detect inhibition of tyrosine hydroxylase, the rate-limiting enzyme in the synthesis of catecholamines. Thirty planaria per dose were exposed to 0 (control), 0.001, 0.01, 0.1, or 1 mM 3-iodo-L-tyrosine (monoiodotyrosine or
Differential regulation of tyrosine hydroxylase in cuticular melanization and innate immunity in the silkworm Bombyx mori
Lee KS, et al.
Journal of Asia-Pacific Entomology, 18(4), 765-770 (2015)
P F Fitzpatrick
Biochemistry, 30(15), 3658-3662 (1991-04-16)
The steady-state kinetic mechanism for rat tyrosine hydroxylase has been determined by using recombinant enzyme expressed in insect tissue culture cells. Variation of any two of the three substrates, tyrosine, 6-methyltetrahydropterin, and oxygen, together at nonsaturating concentrations of the third
Dopamine and serotonin are both required for mate-copying in Drosophila melanogaster
Monier M, et al.
Frontiers in Behavioral Neuroscience, 12(3), 334-334 (2018)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.