G3798
10074-G5
≥98% (HPLC)
동의어(들):
Biphenyl-2-yl-(7-nitrobenzo[1,2,5]oxadiazol-4-yl)amine, N-2-Biphenylyl-7-nitro-2,1,3-benzoxadiazol-4-amine, N-[1,1′-Biphenyl-2-yl]-7-nitro-2,1,3-Benzoxadiazol-4-amine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
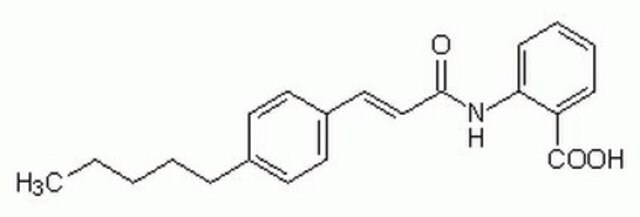
C18H12N4O3
CAS Number:
Molecular Weight:
332.31
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
red
solubility
DMSO: >10 mg/mL
저장 온도
room temp
SMILES string
[O-][N+](=O)c1ccc(Nc2ccccc2-c3ccccc3)c4nonc14
InChI
1S/C18H12N4O3/c23-22(24)16-11-10-15(17-18(16)21-25-20-17)19-14-9-5-4-8-13(14)12-6-2-1-3-7-12/h1-11,19H
InChI key
KMJPYSQOCBYMCF-UHFFFAOYSA-N
생화학적/생리학적 작용
10074-G5 is a c-Myc/Max interaction inhibitor. The c-Myc oncoprotein and its partner Max are intrinsically disordered (ID) monomers that undergo coupled folding and binding upon heterodimerization. 10074-G5, similarly to 10058-F4 (#F3680), specifically inhibits this interaction by binding to c-Myc, thus preventing C-Myc specific DNA binding and target genes regulation. 10074-G5 (2.8 microM) is slightly more potent that 10058-F4 (5.2 microM). It was discovered that 10074-G5 binds to a different specific binding site (region) of C-Myc than 10054-F4. Thus, the compound may become desirable for probing different interactions.
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Chengsheng Wu et al.
BMC cancer, 18(1), 361-361 (2018-04-04)
The phenomenon that malignant cells can acquire stemness under specific stimuli, encompassed under the concept of cancer cell plasticity, has been well-described in epithelial malignancies. To our knowledge, cancer cell plasticity has not yet been described in hematopoietic cancers. To
Quan Yang et al.
Frontiers in immunology, 12, 627072-627072 (2021-03-13)
The accumulation of myeloid-derived suppressor cells (MDSCs) is one of the major obstacles to achieve an appropriate anti-tumor immune response and successful tumor immunotherapy. MDSCs in tumor-bearing hosts are primarily polymorphonuclear (PMN-MDSCs). However, the mechanisms regulating the development of MDSCs
Alina Castell et al.
Scientific reports, 8(1), 10064-10064 (2018-07-04)
MYC is a key player in tumor development, but unfortunately no specific MYC-targeting drugs are clinically available. MYC is strictly dependent on heterodimerization with MAX for transcription activation. Aiming at targeting this interaction, we identified MYCMI-6 in a cell-based protein
Udom Lao-On et al.
Biochimica et biophysica acta. Molecular basis of disease, 1866(3), 165656-165656 (2019-12-25)
Here we showed that the c-Myc oncogene is responsible for overexpression of pyruvate carboxylase (PC) in highly invasive MDA-MB-231 cells. Pharmacological inhibition of c-Myc activity with 10074-G5 compound, resulted in a marked reduction of PC mRNA and protein, concomitant with
Huabo Wang et al.
Oncotarget, 6(18), 15857-15870 (2015-06-04)
The c-Myc (Myc) oncoprotein is deregulated in a large proportion of diverse human cancers. Considerable effort has therefore been directed at identifying pharmacologic inhibitors as potential anti-neoplastic agents. Three such groups of small molecule inhibitors have been described. The first
문서
We present an article about how proliferating cells require the biosynthesis of structural components for biomass production and for genomic replication.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.