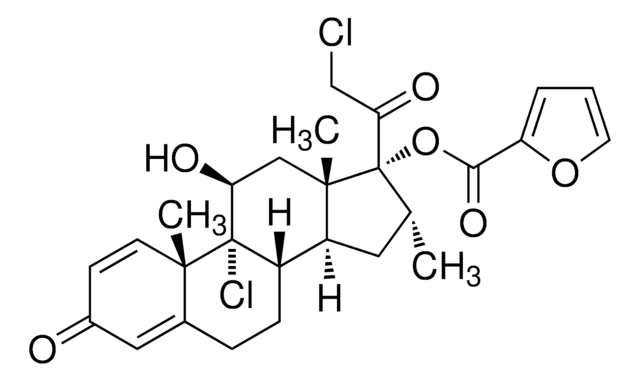
추천 제품
Quality Level
분석
≥97%
주관자
Roche
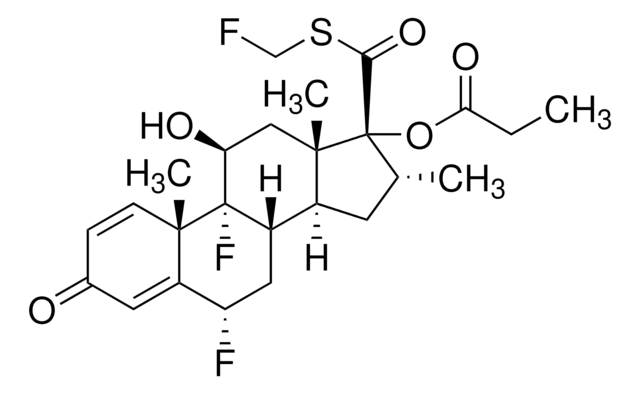
SMILES string
CC1(C)O[C@@H]2CC3C4C[C@H](F)C5=CC(=O)C=CC5(C)C4C(O)CC3(C)[C@@]2(O1)C(=O)CO
InChI
1S/C24H31FO6/c1-21(2)30-19-9-14-13-8-16(25)15-7-12(27)5-6-22(15,3)20(13)17(28)10-23(14,4)24(19,31-21)18(29)11-26/h5-7,13-14,16-17,19-20,26,28H,8-11H2,1-4H3/t13?,14?,16-,17?,19+,20?,22?,23?,24+/m0/s1
InChI key
XSFJVAJPIHIPKU-CCRGDLLISA-N
특징 및 장점
This compound was developed by Roche. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Acute Tox. 1 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
A Varricchio et al.
International journal of immunopathology and pharmacology, 24(2), 401-409 (2011-06-11)
Non-allergic rhinitis (NAR) is a heterogeneous disease, characterized by nasal hyperreactivity and inflammation. Its treatment is still debated, intranasal corticosteroids may be an option. The present study is aimed at evaluating the effect of the use of intranasal flunisolide in
Niels Mygind et al.
Acta oto-laryngologica, 126(10), 1022-1029 (2006-08-23)
The introduction of nasal glucocorticosteroids, 30 years ago, has been the most important therapeutic progress in rhinitis management since the introduction of the first generation of antihistamines. Our knowledge of the mode of action of glucocorticosteroids in the nose has
Jonathan Corren et al.
Clinical therapeutics, 25(3), 776-798 (2003-07-11)
Inhaled corticosteroids are currently recommended as first-line therapy for the long-term control and management of persistent asthma. Flunisolide hydrofluoroalkane (HFA) is a new formulation of the corticosteroid flunisolide that is delivered by a metered-dose inhaler containing an HFA propellant. HFA
G Ciprandi et al.
International journal of immunopathology and pharmacology, 20(4), 833-836 (2008-01-09)
Adenoidal hypertrophy (AH) represents one of the most frequent indications for surgery in children and it has been proposed that treatment with intranasal corticosteroids can decrease the size of AH. Therefore, the aim of the study is to evaluate the
A Varricchio et al.
Journal of biological regulators and homeostatic agents, 23(2), 95-101 (2009-07-11)
Adenoidal hypertrophy (AH) represents one of the most frequent indications for surgery in children. Recently, treatment with intranasal corticosteroids has been suggested to decrease the size of AH. The aim of the study is to evaluate the long-term effect of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.