추천 제품
Quality Level
분석
≥98%
양식
powder
기술
thin layer chromatography (TLC): suitable
solubility
H2O: soluble 50 mg/mL, clear, colorless
저장 온도
−20°C
SMILES string
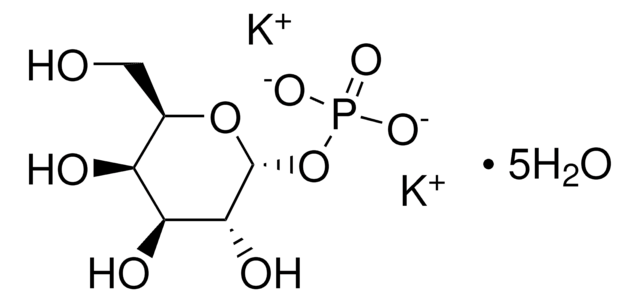
NC1CCCCC1.NC2CCCCC2.C[C@@H]3O[C@H](OP(O)(O)=O)[C@@H](O)[C@H](O)[C@@H]3O
InChI
1S/2C6H13N.C6H13O8P/c2*7-6-4-2-1-3-5-6;1-2-3(7)4(8)5(9)6(13-2)14-15(10,11)12/h2*6H,1-5,7H2;2-9H,1H3,(H2,10,11,12)/t;;2-,3+,4+,5-,6+/m..0/s1
InChI key
FQMPFZHILABVMA-PEJHDPODSA-N
애플리케이션
β-L-Fucose 1-phosphate is suitable as both substrate and product to identify, differentiate and characterize GTP fucose pyrophosphorylase(s) (GFPP; fucose-1-phosphate guanylyltransferases) involved in the formation of the nucleotide-sugar GDP-beta-l-fucose and other fucosylation donor substrates such as 3,3′-Diaminobenzidine (GDP)-β-l-fucose. β-L-Fucose 1-phosphate may be used to generate new fucosylation donor substrates for use in glycan fucosylation research.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
Toshihisa Kotake et al.
The Journal of biological chemistry, 283(13), 8125-8135 (2008-01-18)
Monomeric sugars generated during the metabolism of polysaccharides, glycoproteins, and glycolipids are imported to the cytoplasm and converted to respective nucleotide sugars via monosaccharide 1-phosphates, to be reutilized as activated sugars. Because L-fucose (L-Fuc) is activated mainly in the form
Leonie Engels et al.
Glycobiology, 24(2), 170-178 (2013-11-20)
Fucosyltransferases (FucTs) are essential tools for the synthesis of fucosylated glycoconjugates. Multistep enzyme catalysis of fucosylated glycans is not limited as long as isolated and well-characterized FucTs are available. The present paper introduces a novel bacterial α1,2-FucT of the glycosyltransferase
Bing Ma et al.
Glycobiology, 16(12), 158R-184R (2006-09-16)
Fucosylated carbohydrate structures are involved in a variety of biological and pathological processes in eukaryotic organisms including tissue development, angiogenesis, fertilization, cell adhesion, inflammation, and tumor metastasis. In contrast, fucosylation appears less common in prokaryotic organisms and has been suggested
GDP-L-fucose pyrophosphorylase. Purification, cDNA cloning, and properties of the enzyme.
Pastuszak I, Ketchum C, Hermanson G, et al.
The Journal of Biological Chemistry, 4273, 30165-30174 (1998)
Stephen Quirk et al.
Biochemistry, 44(39), 13172-13178 (2005-09-28)
GTP-l-fucose pyrophosphorylase(GFPP) catalyzes the reversible formation of the nucleotide-sugar GDP-beta-l-fucose from guanosine triphosphate and beta-l-fucose-1-phosphate. The enzyme functions primarily in the mammalian liver and kidney to salvage free fucose during the breakdown of glycoproteins and glycolipids. GFPP shares little primary
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.