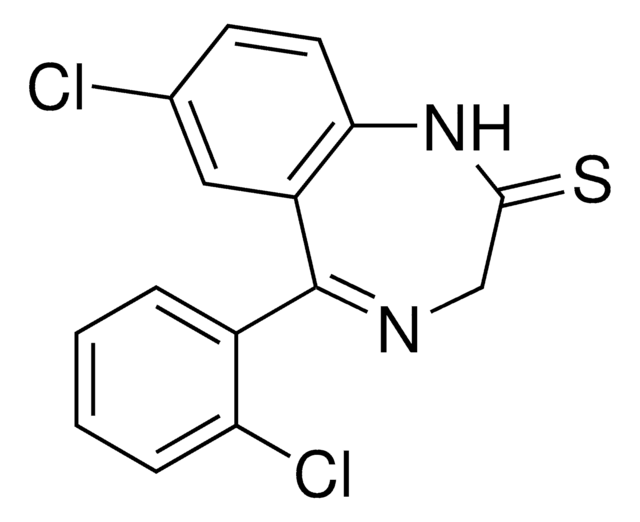
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
solid
색상
white
solubility
DMSO: >20 mg/mL
주관자
Johnson & Johnson
저장 온도
2-8°C
SMILES string
CN1CC(=O)N=C1NC(=O)Nc2cccc(Cl)c2
InChI
1S/C11H11ClN4O2/c1-16-6-9(17)14-10(16)15-11(18)13-8-4-2-3-7(12)5-8/h2-5H,6H2,1H3,(H2,13,14,15,17,18)
InChI key
DWPQODZAOSWNHB-UHFFFAOYSA-N
관련 카테고리
생화학적/생리학적 작용
Fenobam is a potent, selective, noncompetitive glutamate mGluR5 receptor antagonist. Fenobam displays inverse agonist properties; blocks mGluR5 constitutive activity in vitro (IC50 = 87 nM, slightly weaker than MPEP). Fenobam acts at an allosteric modulatory site shared with MPEP and binds the mGlu5 receptor with Kd values of 54 and 31 nM for rat and human receptors, respectively. Fenobam belongs to a structurally different class than MPEP; devoid of GABAergic activity and thus typical benzodiazepine-like side effects; displays anxiolytic activity.
Potent, selective, noncompetitive metabotropic glutamate receptor antagonist (mGluR5). Displays anxiolytic activity.
특징 및 장점
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Glutamate Receptors (G Protein Family) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Johnson & Johnson. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Sajesh P Thomas et al.
Chemical communications (Cambridge, England), 48(85), 10559-10561 (2012-09-25)
Crystal structures of polymorphs and solvatomorphs of the potential anxiolytic drug fenobam exhibit an exclusive preference for one of the two possible tautomeric structures. A novel methodology based on nonlinear optical response has been successfully employed to detect the presence
Michael C Montana et al.
Anesthesiology, 115(6), 1239-1250 (2011-11-01)
The metabotropic glutamate receptor 5 noncompetitive antagonist fenobam is analgesic in rodents. Future development of fenobam as an analgesic in humans will require a favorable long-term treatment profile and a lack of significant deleterious side effects. This study aimed to
Michael C Montana et al.
The Journal of pharmacology and experimental therapeutics, 330(3), 834-843 (2009-06-12)
Metabotropic glutamate receptor subtype 5 (mGlu5) has been demonstrated to play a role in the modulation of numerous nociceptive modalities. When administered via peripheral, intrathecal, or systemic routes, mGlu5 antagonists have analgesic properties in a variety of preclinical pain models.
Lara W Crock et al.
Molecular pain, 8, 20-20 (2012-03-28)
Interstitial cystitis/painful bladder syndrome (IC/PBS), is a severely debilitating chronic condition that is frequently unresponsive to conventional pain medications. The etiology is unknown, however evidence suggests that nervous system sensitization contributes to enhanced pain in IC/PBS. In particular, central nervous
W N Wu et al.
Journal of pharmaceutical sciences, 84(2), 185-189 (1995-02-01)
Fenobam [(Fn); N-(3-chlorophenyl)-N-(4,5-dihydro-1-methyl-4-oxo-1H-imidazole-2-yl)urea] sulfate is a novel agent with potent anxiolytic activity in rats. [14C]Fn sulfate was administered as an oral solution (250 mg/kg) to male Wistar rats, and 52% of the administered dose was excreted in urine (0-5 days).
문서
Sigma-Aldrich offers many products related to G-protein family glutamate receptors for your research needs.
관련 콘텐츠
DISCOVER Bioactive Small Molecules for Neuroscience
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.