E6375
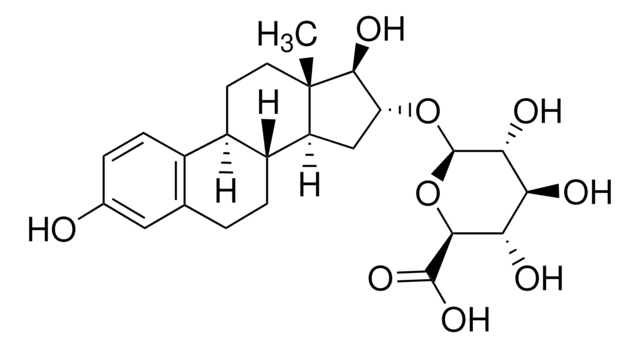
Estriol 3-sulfate sodium salt
≥98% (TLC)
동의어(들):
1,3,5(10)-Estratriene-3,16α,17β-triol 3-sulfate, 3,16α,17β-Trihydroxy-1,3,5(10)-estratriene 3-sulfate
About This Item
추천 제품
Quality Level
분석
≥98% (TLC)
양식
powder
포함
~30% methylglucamine as stabilizer
solubility
methanol: 19.60-20.40 mg/mL, clear, colorless to faintly yellow
응용 분야
diagnostic assay manufacturing
hematology
histology
배송 상태
ambient
저장 온도
room temp
SMILES string
[Na].[H][C@]12CC[C@]3(C)[C@@H](O)[C@H](O)C[C@@]3([H])[C@]1([H])CCc4cc(OS(O)(=O)=O)ccc24
InChI
1S/C18H24O6S.Na.H/c1-18-7-6-13-12-5-3-11(24-25(21,22)23)8-10(12)2-4-14(13)15(18)9-16(19)17(18)20;;/h3,5,8,13-17,19-20H,2,4,6-7,9H2,1H3,(H,21,22,23);;/t13-,14-,15+,16-,17+,18+;;/m1../s1
InChI key
NGMCHEUPKPRHNC-XZEIGEQDSA-N
포장
이미 열람한 고객
문서
Separation of Estriol 3-(β-D-glucuronide) sodium salt; β-Estradiol 3-(β-D-glucuronide) 17-sulfate dipotassium salt; Estriol 3-sulfate sodium salt; β-Estradiol 3,17-disulfate dipotassium salt, ≥95%; β-Estradiol 17-(β-D-glucuronide) sodium salt; β-Estradiol 3-(β-D-glucuronide) sodium salt; Estrone 3-(β-D-glucuronide) sodium salt; β-Estradiol 3-sulfate sodium salt, ≥93%; Estriol, ≥97%; Estrone 3-sulfate sodium salt, contains ~35% Tris as stabilizer; β-Estradiol, ≥98%; α-Estradiol, powder, ≥98% (TLC); Estrone, ≥99%
관련 콘텐츠
The Titan C18 column provided efficient and rapid resolution of thirteen related estrogenic compounds. Ultra Ultra high purity solvents provided robust operation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.