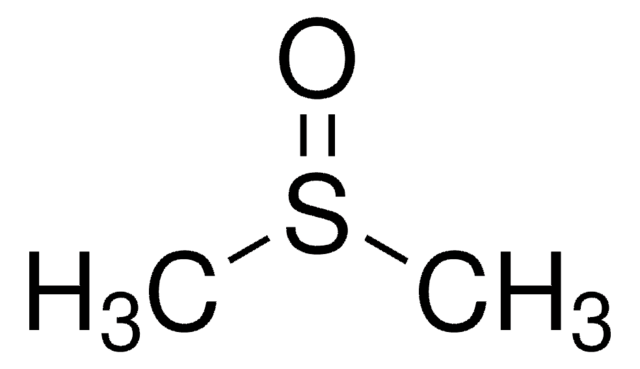
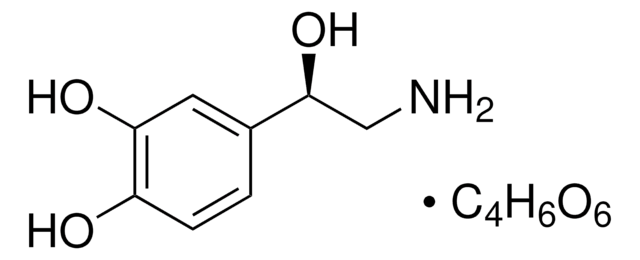
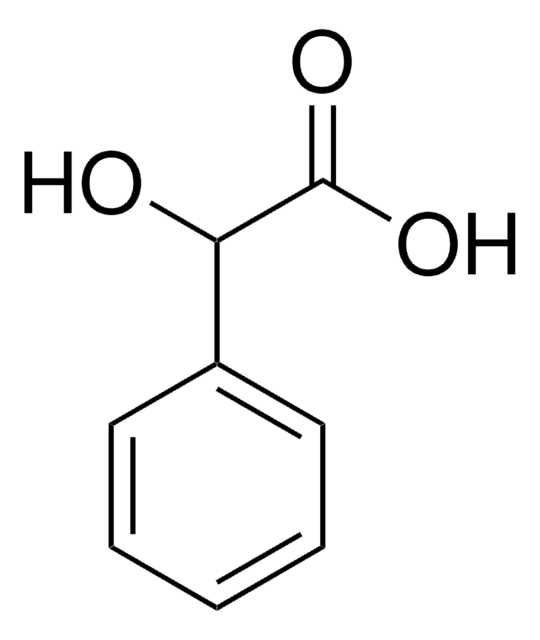
추천 제품
양식
powder
Quality Level
저장 온도
2-8°C
SMILES string
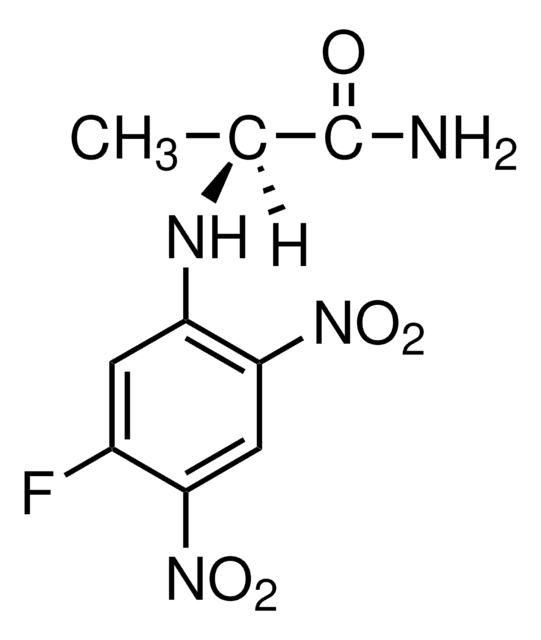
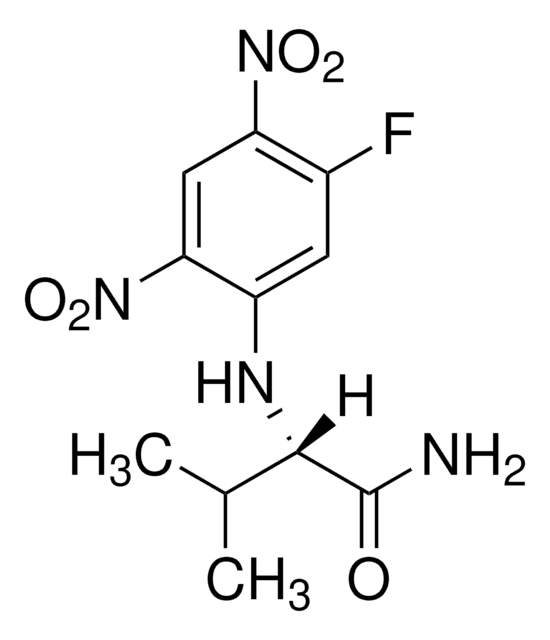
C[C@H](Nc1cc(F)c(cc1[N+]([O-])=O)[N+]([O-])=O)C(N)=O
InChI
1S/C9H9FN4O5/c1-4(9(11)15)12-6-2-5(10)7(13(16)17)3-8(6)14(18)19/h2-4,12H,1H3,(H2,11,15)/t4-/m0/s1
InChI key
NEPLBHLFDJOJGP-BYPYZUCNSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
N-α-(2,4-Dinitro-5-fluorophenyl)-L-alaninamide is suitable for use for the derivatization of amino acids. glutamate and analine present in cell wall. Derivatized D- and L-amino acids can be resolved and quantitated by HPLC.
생화학적/생리학적 작용
N-α-(2,4-Dinitro-5-fluorophenyl)-L-alaninamide (FDAA) is a chiral derivatizing agent and is used routinely to improve the detection of underivatized amino acids in high performance liquid chromatography. Its usage is effective in separating stereoisomer.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
The functional dlt operon of Clostridium butyricum controls the D-alanylation of cell wall components and influences cell septation and vancomycin-induced lysis
Wydau-Dematteis S, et al.
Anaerobe, 35, 105-114 (2015)
High concentrations of D-amino acids in human gastric juice
Nagata Y, et al.
Amino Acids, 32(1), 137-140 (2007)
A comparison of the direct and indirect LC methods for separating enantiomers of unusual glycine and alanine amino acid analogues
Peter A, et al.
Chromatographia, 56(1), S79-S89 (2002)
C3 and 2D C3 Marfey?s methods for amino acid analysis in natural products
Vijayasarathy S, et al.
Journal of Natural Products, 79(2), 421-427 (2016)
Purification of branched-chain amino acid aminotransferase from Helicobacter pylori NCTC 11637
Saito M, et al.
Amino Acids, 33(3), 445-449 (2007)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.