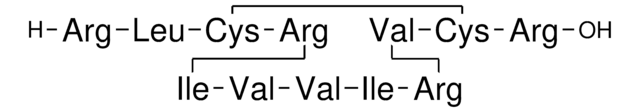추천 제품
생물학적 소스
Streptomyces cinnamoneus
Quality Level
분석
≥95% (HPLC)
양식
solid
solubility
DMSO: 10 mg/mL
acetonitrile: water (1:1): 5 mg/mL (requires heating)
항생제 활성 스펙트럼
fungi
동작 모드
cell membrane | interferes
저장 온도
2-8°C
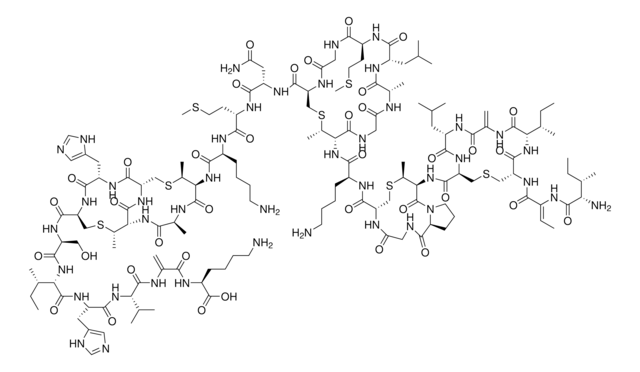
SMILES string
S1[C@@H](C2NC(=O)[C@@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H]3NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H]5NC(=O)[C@@H](NC(=O)[C@H]7N(CCC7)C(=O)CNC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C1)N)CCCN\C(=N/[H])\N)CCC(=O)N)CS
InChI
1S/C89H125N25O25S3/c1-43(2)66-84(133)109-59-41-140-40-58-79(128)108-60-42-142-45(4)68(86(135)105-55(75(124)110-66)34-48-22-12-7-13-23-48)111-76(125)54(33-47-20-10-6-11-21-47)104-82(131)61-26-17-31-114(61)65(118)38-98-72(121)53(32-46-18-8-5-9-19-46)103-78(127)57(106-80(60)129)36-95-29-15-14-24-52(87(136)137)102-85(134)67(112-77(126)56(35-63(92)116)99-64(117)37-97-83(132)69(113-81(59)130)70(119)88(138)139)44(3)141-39-49(90)71(120)100-50(25-16-30-96-89(93)94)73(122)101-51(74(123)107-58)27-28-62(91)115/h5-13,18-23,43-45,49-61,66-70,95,119H,14-17,24-42,90H2,1-4H3,(H2,91,115)(H2,92,116)(H,97,132)(H,98,121)(H,99,117)(H,100,120)(H,101,122)(H,102,134)(H,103,127)(H,104,131)(H,105,135)(H,106,129)(H,107,123)(H,108,128)(H,109,133)(H,110,124)(H,111,125)(H,112,126)(H,113,130)(H,136,137)(H,138,139)(H4,93,94,96)/t44-,45?,49+,50+,51+,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,66+,67?,68+,69+,70-/m1/s1
InChI key
QJDWKBINWOWJNZ-IDGBIKHQSA-N
Amino Acid Sequence
일반 설명
애플리케이션
생화학적/생리학적 작용
Cinnamycin, like other lantibiotics, was also reported to inhibit phospholipase A2 (PLA2). It was suggested as an alternative treatment for atherosclerosis through its ability to inhibit PLA2 by binding to its substrate PE. Moreover, Cinnamycin was found to inhibit Herpes simplex virus (HSV-1) activity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
문서
With bacterial resistance and emerging infectious diseases becoming potential threats to humans, ribosomally synthesized antimicrobial peptides have become a promising focus area in antibiotic research.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.