모든 사진(1)
About This Item
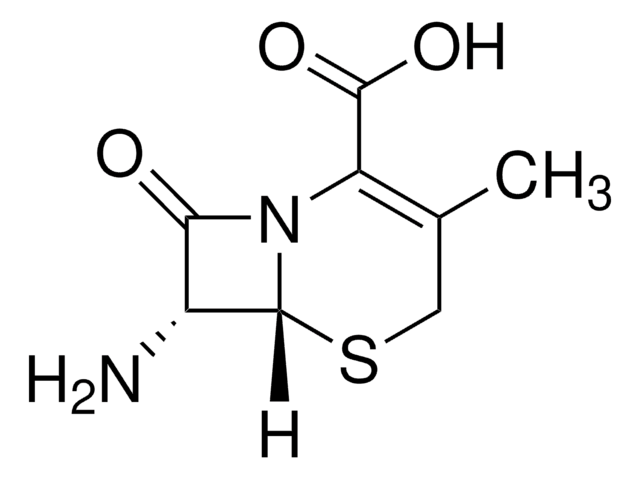
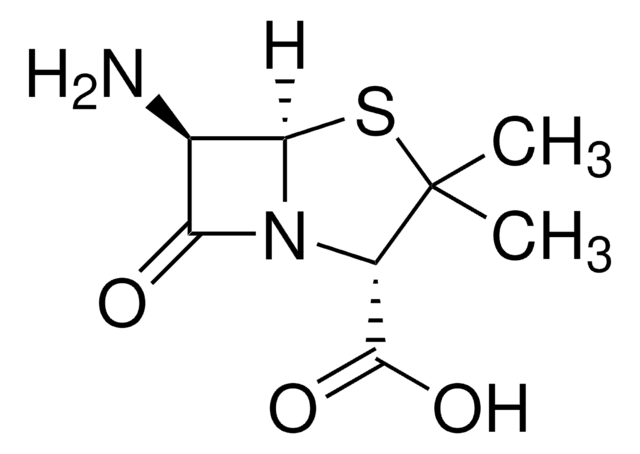
실험식(Hill 표기법):
C8H10N2O3S
CAS Number:
Molecular Weight:
214.24
EC Number:
MDL number:
UNSPSC 코드:
51102829
PubChem Substance ID:
NACRES:
NA.85
추천 제품
양식
powder or crystals
Quality Level
항생제 활성 스펙트럼
Gram-positive bacteria
동작 모드
cell wall synthesis | interferes
저장 온도
2-8°C
SMILES string
CC1=C(N2[C@H](SC1)[C@H](N)C2=O)C(O)=O
InChI
1S/C8H10N2O3S/c1-3-2-14-7-4(9)6(11)10(7)5(3)8(12)13/h4,7H,2,9H2,1H3,(H,12,13)/t4-,7-/m1/s1
InChI key
NVIAYEIXYQCDAN-CLZZGJSISA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chemical structure: ß-lactam
애플리케이션
7-Aminodesacetoxycephalosporanic acid is used in the synthesis of cephalosporins and for bioconversion studies .
생화학적/생리학적 작용
7-ADCA is produced from penicillin G made by Penicillium chrysogenum involving several polluting chemical steps followed by enzymatic deacylation using penicillin acylase .
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
기타 정보
Keep container tightly closed in a dry and well-ventilated place.
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
이미 열람한 고객
Mutational analysis of a key residue in the substrate specificity of a cephalosporin acylase.
Linda G. Otten, Charles F. Sio, et al.
Chembiochem, 6, 820-825 (2004)
Linda G Otten et al.
The Journal of biological chemistry, 277(44), 42121-42127 (2002-08-29)
Using directed evolution, we have selected an adipyl acylase enzyme that can be used for a one-step bioconversion of adipyl-7-aminodesacetoxycephalosporanic acid (adipyl-7-ADCA) to 7-ADCA, an important compound for the synthesis of semisynthetic cephalosporins. The starting point for the directed evolution
A N Ivankin et al.
Prikladnaia biokhimiia i mikrobiologiia, 36(3), 303-306 (2000-06-27)
The use of peptide hydrolase (EC 3.4.13.1) from Xanthomonas rubrilineans for synthesis of the antibiotic cephalexin from 7-aminodesacetoxycephalosporanic acid was studied. The optimum conditions for production of cephalexin were determined, and the yield exceeded 80%. A method for monitoring the
C G P H Schroën et al.
Biotechnology and bioengineering, 80(2), 144-155 (2002-09-05)
Integrated process concepts for enzymatic cephalexin synthesis were investigated by our group, and this article focuses on the integration of reactions and product removal during the reactions. The last step in cephalexin production is the enzymatic kinetic coupling of activated
C G Schroën et al.
Biotechnology and bioengineering, 70(6), 654-661 (2000-11-07)
One of the building blocks of cephalosporin antibiotics is 7-amino-deacetoxycephalosporanic acid (7-ADCA). It is currently produced from penicillin G using an elaborate chemical ring-expansion step followed by an enzyme-catalyzed hydrolysis. However, 7-ADCA-like components can also be produced by direct fermentation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.