추천 제품
생물학적 소스
synthetic (organic)
Quality Level
분석
≥98% (TLC)
양식
powder
약물 제어
regulated under CDSA - not available from Sigma-Aldrich Canada
기술
GC/MS: suitable
mp
140-141 °C (lit.)
solubility
ethanol: 19.60-20.40 mg/mL, clear, colorless
배송 상태
ambient
저장 온도
room temp
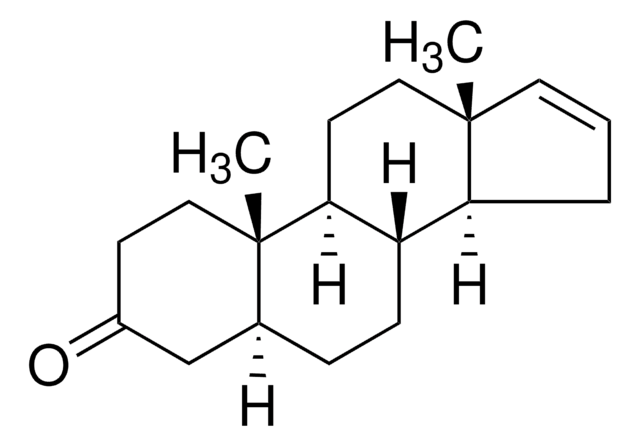
SMILES string
C[C@]12CC[C@H]3[C@@H](CC[C@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC=C2
InChI
1S/C19H30O/c1-18-9-3-4-16(18)15-6-5-13-12-14(20)7-11-19(13,2)17(15)8-10-18/h3,9,13-17,20H,4-8,10-12H2,1-2H3/t13-,14+,15-,16-,17-,18-,19-/m0/s1
InChI key
KRVXMNNRSSQZJP-PHFHYRSDSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
5-α-androst-16-en-3-α-ol (androstenol) is an androgen believed to act as a pheromone. Androstenol has been used in a study to develop a combined gas chromatography/thermal conversion/isotope ratio mass spectrometry (GC/TC/IRMS) method for D/H ratio determination of endogenous urinary steroids.
생화학적/생리학적 작용
5alpha-androst-16-en-3alpha-ol (androstenol), is an androgen believed to act as a pheromone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Thomas Piper et al.
Rapid communications in mass spectrometry : RCM, 23(13), 1917-1926 (2009-05-23)
The development and application of a combined gas chromatography/thermal conversion/isotope ratio mass spectrometry (GC/TC/IRMS) method for D/H ratio determination of endogenous urinary steroids are presented. The key element in sample preparation was the consecutive cleanup with high-performance liquid chromatography of
Jeremy Vincent et al.
Acta crystallographica. Section F, Structural biology and crystallization communications, 61(Pt 1), 156-159 (2006-03-02)
The constitutive androstane receptor (CAR) is a member of the nuclear receptor superfamily. In contrast to classical nuclear receptors, which possess small-molecule ligand-inducible activity, CAR exhibits constitutive transcriptional activity in the apparent absence of ligand. CAR is among the most
E L Hurden et al.
The Journal of endocrinology, 103(2), 179-186 (1984-11-01)
Three mature Large White boars were anaesthetized and received [7(n)-3H]pregnenolone by continuous infusion into right and left spermatic arteries for up to 180 min. Spermatic venous blood flow was measured by separate timed collections of completely diverted outflow from each
J J Cowley et al.
The Journal of steroid biochemistry and molecular biology, 39(4B), 647-659 (1991-10-01)
Student volunteers (38 of each sex) were exposed unknowingly overnight to the vapour of pheromonally active substances and compared with controls. The substances were either 5 alpha-16-androsten-3 alpha-ol (androstenol, occurring in human underarm sweat, and known to be pheromonally active
[Synthesis of mammalian pheromone 5 alpha-androst-16-en-3-one and 5 alpha-Androst-16-en-3 alpha-ol].
G D Han et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 17(9), 696-698 (1982-09-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.