추천 제품
일반 설명
L-Alanine Dehydrogenase has a N-terminal substrate-binding domain and a C-terminal NAD-binding domain.
애플리케이션
L-Alanine Dehydrogenase from Bacillus subtilis has been used in the carbon nanotube columns for H2-driven biocatalysis hydrogenation studies.
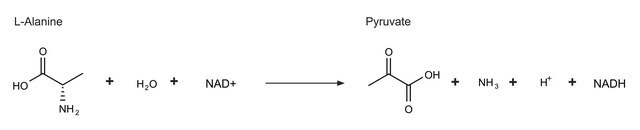
L-Alanine dehydrogenase converts L-alanine to pyruvate and ammonium. L-Alanine dehydrogenase from Bacillus subtilis may be used to study enzyme inactivation and protection .
생화학적/생리학적 작용
L-Alanine Dehydrogenase is essential for sporulation in Bacillus subtilis.
L-Alanine dehydrogenase is a stereospecific dehydrogenase that catalyzes the reversible deamination of L-alanine to pyruvate and ammonium. It is important for the generation of pyruvate during sporulation. L-Alanine dehydrogenase from Bacillus subtilis has a predominately ordered kinetic mechanism in which NAD binds before L-alanine. Subsequently, ammonia, pyruvate and NADH are released in that specific order. Optimal pH for the amination reaction is 8.8-9.0, whereas it is 10-10.5 for the deamination reaction. The enzyme is inactivated by divalent metal ions and p-chloromercuribenzoate, mercuric ion being most effective. The inactivation may be reversed by L- or D-cysteine.
단위 정의
One unit will convert 1.0 μmole of L-alanine to pyruvate and NH3 per min at pH 10.0 at 25 °C.
물리적 형태
Suspension in 2.4 M (NH4)2SO4 solution, pH 7.0
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
H 2-Driven biocatalytic hydrogenation in continuous flow using enzyme-modified carbon nanotube columns
Zor C, et al.
Chemical Communications (Cambridge, England), 53(71), 9839-9841 (2017)
Alanine dehydrogenase (ald) is required for normal sporulation in Bacillus subtilis.
Siranosian K, et al.
Journal of Bacteriology, 175(21), 6789-6796 (1993)
Domain motions and functionally-key residues of l-alanine dehydrogenase revealed by an elastic network model
Li XY, et al.
International Journal of Molecular Sciences, 16(12), 29383-29397 (2015)
D Delforge et al.
The Journal of biological chemistry, 272(4), 2276-2284 (1997-01-24)
L-Alanine dehydrogenase from Bacillus subtilis was inactivated with two different lysine-directed chemical reagents, i.e. 2,4, 6-trinitrobenzenesulfonic acid and N-succinimidyl 3-(2-pyridyldithio)propionate. In both cases, the inactivation followed pseudo first-order kinetics, with a 1:1 stoichiometric ratio between the reagent and the enzyme
Xueli Zhang et al.
Applied microbiology and biotechnology, 77(2), 355-366 (2007-09-18)
Escherichia coli W was genetically engineered to produce L: -alanine as the primary fermentation product from sugars by replacing the native D: -lactate dehydrogenase of E. coli SZ194 with alanine dehydrogenase from Geobacillus stearothermophilus. As a result, the heterologous alanine
문서
Instructions for working with enzymes supplied as ammonium sulfate suspensions
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.