모든 사진(1)
About This Item
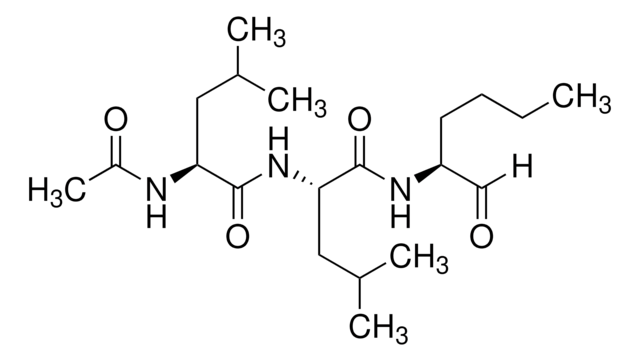
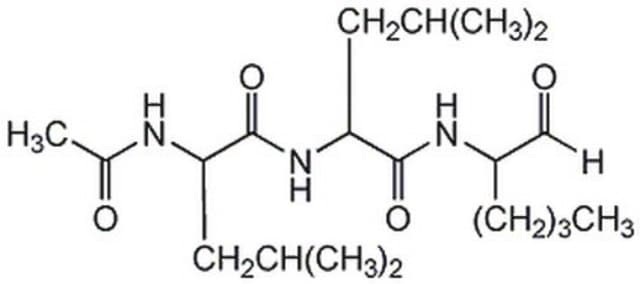
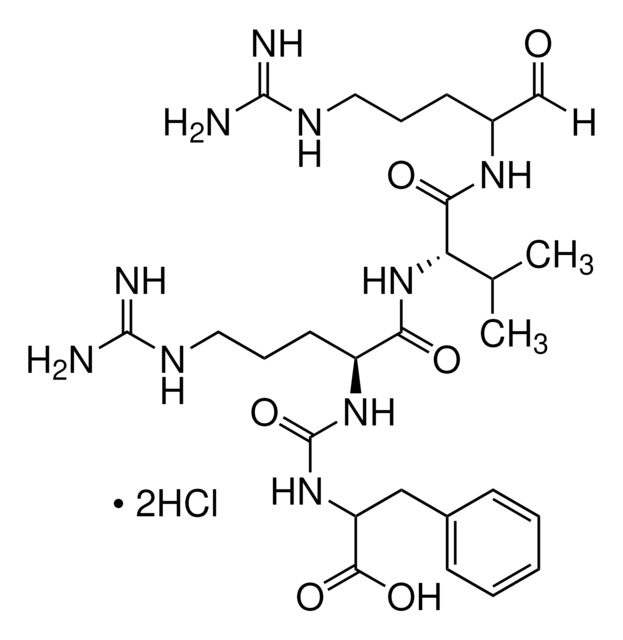
실험식(Hill 표기법):
C19H35N3O4S
CAS Number:
Molecular Weight:
401.56
Beilstein:
7693643
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
분석
≥95% (HPLC)
Quality Level
양식
powder
색상
white
solubility
ethanol: 20 mg/mL
저장 온도
−20°C
SMILES string
[H]C(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(C)=O
InChI
1S/C19H35N3O4S/c1-12(2)9-16(20-14(5)24)19(26)22-17(10-13(3)4)18(25)21-15(11-23)7-8-27-6/h11-13,15-17H,7-10H2,1-6H3,(H,20,24)(H,21,25)(H,22,26)/t15-,16-,17-/m0/s1
InChI key
RJWLAIMXRBDUMH-ULQDDVLXSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Calpain Inhibitor II has been used in western blotting. It has also been used to study the mechanism of Spy1A (a cyclin-like protein) degradation during cell cycle.
Calpain inhibitor II is a cell-permeable peptide that restricts the activity of calpain, cathepsin L and cathepsin B. Calpain inhibitor II also prevents the methylmercury-induced cell death of cultured rat cerebellar neurons.
생화학적/생리학적 작용
Calpain is a cysteine protease expressed in the nervous system. Calpain exhibits a calcium-dependent enzyme activity. In mice models, inhibition of calpain prevents leukocyte infiltration induced by angiotensin II and also attenuates prevascular inflammation.
기타 정보
Formerly CAS# 136632-32-1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Keduo Qian et al.
Bioorganic & medicinal chemistry letters, 21(19), 5944-5947 (2011-08-23)
In this study, 22 new betulinic acid (BA) derivatives were synthesized and tested for their inhibition of the chymotrypsin-like activity of 20S proteasome. From the SAR study, we concluded that the C-3 and C-30 positions are the pharmacophores for increasing
The cyclin-dependent kinase activator, Spy1A, is targeted for degradation by the ubiquitin ligase NEDD4
Al Sorkhy M, et al.
The Journal of Biological Chemistry, 284(5), 2617-2627 (2009)
Zhao Dang et al.
Bioorganic & medicinal chemistry letters, 21(7), 1926-1928 (2011-03-11)
A new class of proteasome inhibitors was synthesized using lithocholic acid as a scaffold. Modification at the C-3 position of lithocholic acid with a series of acid acyl groups yielded compounds with a range of potency on proteasome inhibition. Among
Spontaneous epileptiform discharges in a mouse model of Alzheimer's disease are suppressed by antiepileptic drugs that block sodium channels
Ziyatdinova S, et al.
Epilepsy Research (2011)
Aneurysm: New Insights for the Healthcare Professional, 56-57 (2013)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.