A2736
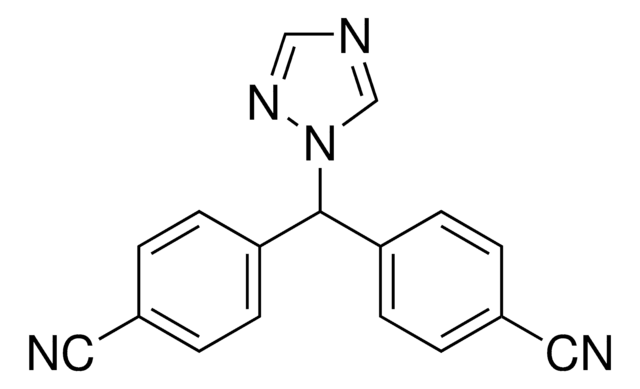
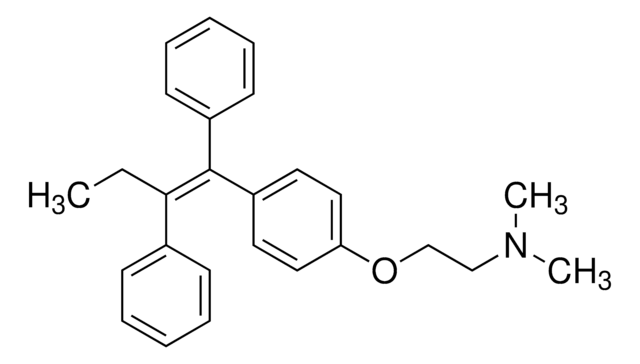
Anastrozole
≥98% (HPLC)
동의어(들):
2;2"-[5-(1H-1;2;4-Triazol-1-ylmethyl)-1, 3-Phenylene]bis(2-methyl-propiononitrile, Arimidex, ICI-D1033, ZD1033, a,a,a′,a′-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C17H19N5
CAS Number:
Molecular Weight:
293.37
MDL number:
UNSPSC 코드:
51111800
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
solid
solubility
DMSO: 40 mg/mL
주관자
AstraZeneca
저장 온도
room temp
SMILES string
[n]2(ncnc2)Cc1cc(cc(c1)C(C)(C)C#N)C(C)(C)C#N
InChI
1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3
InChI key
YBBLVLTVTVSKRW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Anastrozole (aromatase inhibitor) has been used:
- as a positive control in DNA fragmentation (ladder) assay
- to investigate its effects along with extra virgin olive oil and its major fatty acid component (omega-9 OA) in estrogen receptor positive mammary adenocarcinoma cells
- to study its effects on viability, cell proliferation and apoptosis in Glioblastoma multiforme model in vivo
생화학적/생리학적 작용
Anastrozole is a nonsteroidal aromatase inhibitor.
Anastrozole, which contains a triazole functional group, reversibly binds to the cytochrome P-450 component of aromatase. Binding interferes with the catalytic properties of aromatase, which results in inhibition of estrogen synthesis.
The aromatase enzyme converts adrenal androgens to estrogen; this enzymatic activity is the primary source of estrogen production in postmenopausal women. One treatment for estrogen receptor-positive breast cancer in postmenopausal women is through inhibition of aromatase. Anastrozole is a nonsteroidal, benzyl-triazole derivative that inhibits aromatase through competitive inhibition and is used to treat estrogen receptor-positive breast cancer. This compound is considered a third-generation, Type II aromatase inhibitor because it is more selective and less effective (if at all) on other steroidal hormones than first and second generation inhibitors.
특징 및 장점
This compound is featured on the Nuclear Receptors (Steroids) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by AstraZeneca. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Novel acylated steroidal glycosides from Caralluma tuberculata induce caspase-dependent apoptosis in cancer cells
Waheed A, et al.
Journal of Ethnopharmacology, 137(3), 1189-1196 (2011)
Yanyan Hong et al.
Steroids, 76(8), 802-806 (2011-03-23)
Aromatase is the rate-limiting enzyme in estrogen biosynthesis. As a cytochrome P450, it utilizes electrons from NADPH-cytochrome P450 reductase (CPR) to produce estrogen from androgen. Estrogen is a key factor in the promotion of hormone-dependent breast cancer growth. Aromatase inhibitors
P Dubsky et al.
Annals of oncology : official journal of the European Society for Medical Oncology, 24(3), 640-647 (2012-10-05)
In early estrogen receptor (ER)-positive/HER2-negative breast cancer, the decision to administer chemotherapy is largely based on prognostic criteria. The combined molecular/clinical EndoPredict test (EPclin) has been validated to accurately assess prognosis in this population. In this study, the clinical relevance
F Boccardo et al.
European journal of cancer (Oxford, England : 1990), 49(7), 1546-1554 (2013-02-19)
The Italian Tamoxifen Anastrozole (ITA) trial investigated the efficacy of switching to anastrozole for women who were already on adjuvant tamoxifen since 2-3years. Relapse-free survival (RFS) was the primary end-point; event-free survival (EFS), overall survival (OS) and safety were secondary
P I Petkov et al.
SAR and QSAR in environmental research, 20(7-8), 657-678 (2009-12-22)
Cytochrome P450 aromatase is a key steroidogenic enzyme that converts androgens to estrogens in vertebrates. There is much interest in aromatase inhibitors (AIs) both because of their use as pharmaceuticals in the treatment of estrogen-sensitive breast cancers, and because a
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.