추천 제품
제품명
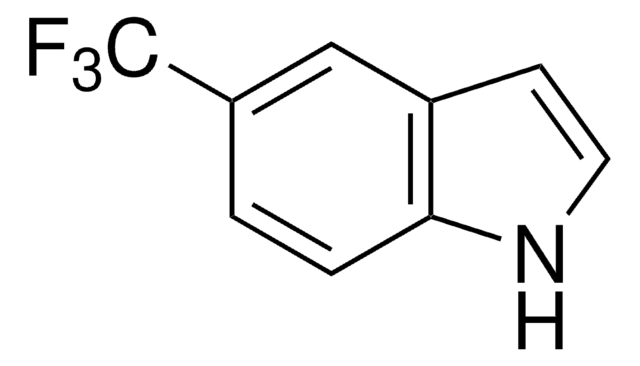
DL-7-Azatryptophan hydrate,
분석
≥98% (TLC)
양식
powder
색상
white to off-white
저장 온도
−20°C
SMILES string
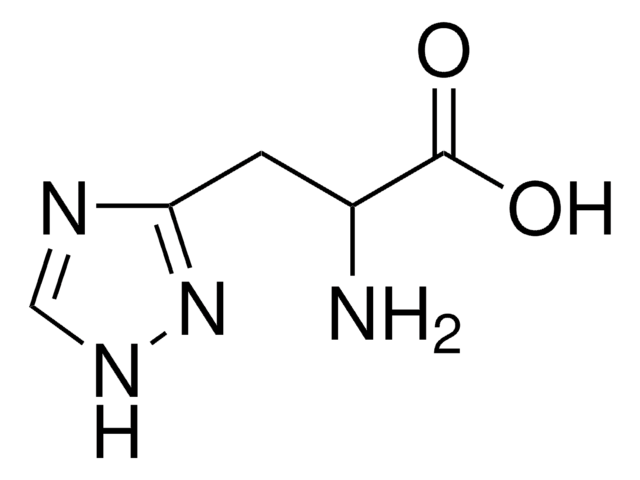
O.NC(Cc1c[nH]c2ncccc12)C(O)=O
InChI
1S/C10H11N3O2.H2O/c11-8(10(14)15)4-6-5-13-9-7(6)2-1-3-12-9;/h1-3,5,8H,4,11H2,(H,12,13)(H,14,15);1H2
InChI key
PXDRHYQAIUZKHN-UHFFFAOYSA-N
생화학적/생리학적 작용
DL-7-Azatryptophan is a racemic mixture of D- and L-7-azatryptophan which together with L-tryptophan is a synergistic inducer of tryptophan oxygenase of Pseudomonas acidovorans. DL-7-Azatryptophan inhibits photosynthetic carbon assimilation, photosynthetic oxygen evolution and nitrogen metabolism in Anabaena sp. Strain 1F, a marine filamentous, heterocystous cyanobacterium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Julie M G Rogers et al.
Analytical biochemistry, 399(2), 182-189 (2009-12-29)
Fluorescence resonance energy transfer (FRET) provides a powerful means to study protein conformational changes. However, the incorporation of an exogenous FRET pair into a protein could lead to undesirable structural perturbations of the native fold. One of the viable strategies
Arnaldo L Serrano et al.
The journal of physical chemistry. B, 116(35), 10631-10638 (2012-08-16)
The villin headpiece subdomain (HP35) has become one of the most widely used model systems in protein folding studies, due to its small size and ultrafast folding kinetics. Here, we use HP35 as a test bed to show that the
P Soumillion et al.
Protein engineering, 8(5), 451-456 (1995-05-01)
The phage lambda lysozyme (lambda L) contains four tryptophans. These have been efficiently replaced by 7-azatryptophan (7aW) through biosynthetic incorporation into the overexpressed protein. Comparative analysis of the effect of temperature or pH on the fluorescence of the wild-type lambda
Fernando Formaggio et al.
Advances in experimental medicine and biology, 527, 731-737 (2004-06-23)
Aal and 7-Atrp, quasi-isosteric with Trp, have been inserted together with a TOAC residue in two 3(10)-helical, model hexapeptides. The interaction of photoexcited AA1 and 7-Atrp with the nitroxide group of TOAC was investigated by time resolved EPR. Both peptides
Vasant Muralidharan et al.
Journal of the American Chemical Society, 126(43), 14004-14012 (2004-10-28)
An integrated approach is described that allows the domain-specific incorporation of optical probes into large recombinant proteins. The strategy is the combination of two existing techniques, expressed protein ligation (EPL) and in vivo amino acid replacement of tryptophans with tryptophan
Chromatograms
application for HPLC자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)