추천 제품
Quality Level
분석
≥75% (HPLC)
양식
liquid
광학 활성
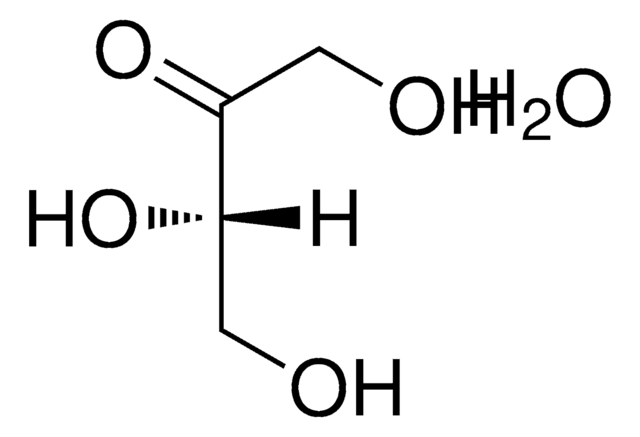
[α]/D 12.0±2.0°, c = 2 in H2O (24 h)
불순물
≤23% water
색상
light yellow
저장 온도
room temp
SMILES string
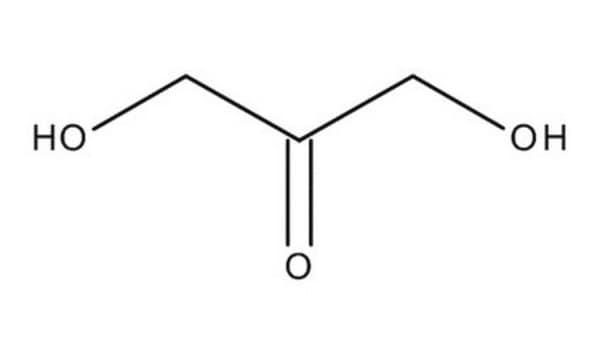
OC[C@H](O)C(=O)CO
InChI
1S/C4H8O4/c5-1-3(7)4(8)2-6/h3,5-7H,1-2H2/t3-/m0/s1
InChI key
UQPHVQVXLPRNCX-VKHMYHEASA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
L-(+)-Erythrulose is used as a tanning agent in the cosmetics industry and a source of chiral ethyl ketones used in aldo reaction organic synthesis.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Gloves
이미 열람한 고객
Santiago Díaz-Oltra et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(30), 9240-9254 (2008-08-30)
Both matched and mismatched diastereoselection have been observed in aldol reactions of a boron enolate of a protected L-erythrulose derivative with several chiral alpha-fluoro and alpha-amino aldehydes. Strict adherence to the Felkin-Anh model for the respective transition structures does not
Santiago Díaz-Oltra et al.
The Journal of organic chemistry, 70(20), 8130-8139 (2005-11-10)
[Chemical reaction: See text] Both matched and mismatched diastereoselections have been observed in the aldol reactions of a range of chiral aldehydes with the dicyclohexylboron enolate of a chiral ethyl ketone related to L-erythrulose. As was previously observed in the
J Alberto Marco et al.
The Journal of organic chemistry, 68(22), 8577-8582 (2003-10-25)
Both matched and mismatched diastereoselections have been observed in aldol reactions of the B,B-dicyclohexylboron enolate of a protected l-erythrulose derivative with a range of chiral aldehydes. The stereochemical outcome of reactions with alpha-methyl aldehydes can be adequately explained within the
G L Simpson et al.
Biochimica et biophysica acta, 1501(1), 12-24 (2000-03-23)
The degradation of L-ascorbate (AsA) and its primary oxidation products, L-dehydroascorbate (DHA) and 2,3-L-diketogulonate (2, 3-DKG) were studied under physiological conditions. Analysis determined that L-erythrulose (ERU) and oxalate were the primary degradation products of ASA regardless of which compound was
Xingxing Zou et al.
Journal of agricultural and food chemistry, 65(35), 7721-7725 (2017-07-15)
L-erythrose, a rare aldotetrose, possesses various pharmacological activities. However, efficient L-erythrose production is challenging. Currently, L-erythrose is produced by a two-step fermentation process from erythritol. Here, we describe a novel strategy for the production of L-erythrose in Gluconobacter oxydans (G.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.