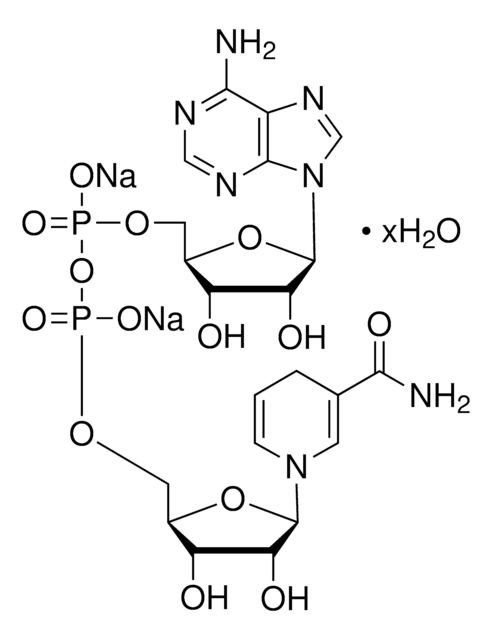
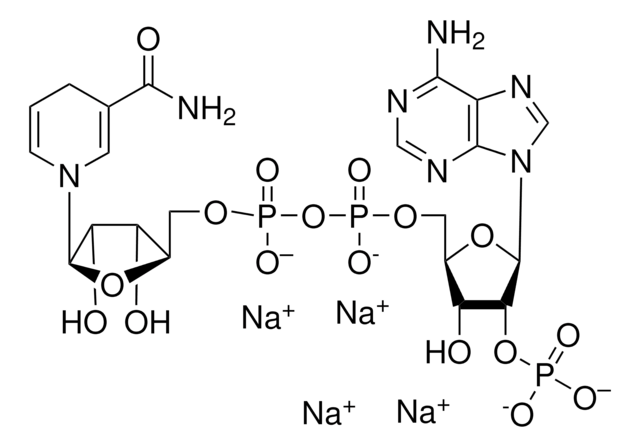
추천 제품
생물학적 소스
equine
Quality Level
재조합
expressed in E. coli
설명
Isozyme E sequence
양식
lyophilized powder
특이 활성도
≥0.5 U/mg
색상
white
light yellow
pH
7
solubility
water: 5 mg/mL
응용 분야
life science and biopharma
저장 온도
−20°C
유전자 정보
equine ... ADH1(111772995)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Research Area: Neuroscience
Alcohol dehydrogenase is a zinc metalloprotein that forms five classes of isoenzymes through the dimerization of eight different subunits.
Alcohol dehydrogenase is a zinc metalloprotein that forms five classes of isoenzymes through the dimerization of eight different subunits.
애플리케이션
Alcohol Dehydrogenase equine has been used in in vitro alcohol dehydrogenase (Adh) assay.
생화학적/생리학적 작용
Alcohol dehydrogenase catalyzes the oxidative conversion of alcohol into aldehyde. It has a homodimeric structure with a co-enzyme binding domain at the C-terminal and an N-terminal catalytic domain. The active site is located at the interdomain cleft. Binding of NAD+ in the active site causes conformational changes which create the binding site for the alcohol substrate.
Horse liver alcohol dehydrogenase (HL-ADH) is an enzyme with broad specificity, capable of catalyzing the reversible oxidation of a wide variety of primary and secondary alcohols to form their corresponding aldehydes and ketones. Moreover, alcohol dehydrogenase can oxidize ethanol while simultaneously reducing nicotinamide adenine dinucleotide (NAD+) to NADH. Previous studies have demonstrated that ADH and ALDH variants can influence alcohol dependence. Additionally, the ADH genotype has been linked to lacunar infarction and neuropsychiatric diseases.
단위 정의
1 U corresponds to the amount of enzyme which reduces 1 μmol benzaldehyde per minute at pH 7.0 and 30 °C.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Ioanna A Gorbunova et al.
The journal of physical chemistry. B, 125(34), 9692-9707 (2021-08-20)
The dynamics of polarized fluorescence in NADH in alcohol dehydrogenase (ADH) in buffer solution has been studied using the TCSPC spectroscopy. A global fit procedure was used for determination of the fluorescence parameters from experiment. The interpretation of the results
Steven Hayward et al.
Biophysical journal, 91(5), 1823-1831 (2006-05-23)
Horse liver alcohol dehydrogenase is a homodimer, the protomer having a coenzyme-binding domain and a catalytic domain. Using all available x-ray structures and 50 ns of molecular dynamics simulations, we investigated the mechanism of NAD+-induced domain closure. When the well-known
Structure of a triclinic ternary complex of horse liver alcohol dehydrogenase at 2.9 A resolution.
H Eklund et al.
Journal of molecular biology, 146(4), 561-587 (1981-03-15)
F Colonna-Cesari et al.
The Journal of biological chemistry, 261(32), 15273-15280 (1986-11-15)
A study of the hinge bending mode in the enzyme liver alcohol dehydrogenase is made by use of empirical energy functions. The enzyme is a dimer, with each monomer composed of a coenzyme binding domain and a catalytic domain with
H Eklund et al.
Biochemistry, 23(25), 5982-5996 (1984-12-04)
The binding of NAD to liver alcohol dehydrogenase has been studied in four different ternary complexes by using crystallographic methods. These complexes crystallize isomorphously in a triclinic crystal form which contains the whole dimer of the enzyme in the asymmetric
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.