19176
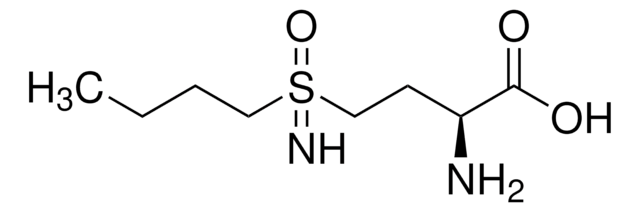
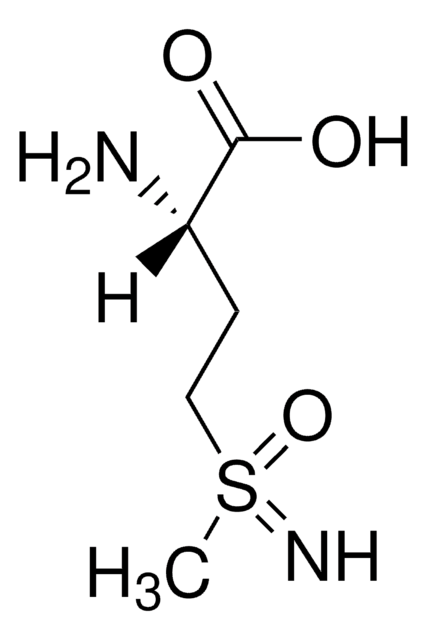
DL-Buthionine-sulfoximine
≥99.0% (TLC)
동의어(들):
DL-Buthionine (S,R)-sulfoximine, BSO, Buthionine sulfoximine, Butionine sulfoximine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C8H18N2O3S
CAS Number:
Molecular Weight:
222.31
Beilstein:
2367136
MDL number:
UNSPSC 코드:
12352202
PubChem Substance ID:
NACRES:
NA.77
추천 제품
생물학적 소스
synthetic
Quality Level
분석
≥99.0% (TLC)
양식
powder or crystals
mp
215 °C
solubility
H2O: 50 mg/mL, clear to almost clear, colorless
저장 온도
2-8°C
SMILES string
CCCCS(=N)(=O)CCC(N)C(O)=O
InChI
1S/C8H18N2O3S/c1-2-3-5-14(10,13)6-4-7(9)8(11)12/h7,10H,2-6,9H2,1H3,(H,11,12)
InChI key
KJQFBVYMGADDTQ-UHFFFAOYSA-N
관련 카테고리
애플리케이션
DL-Buthionine-sulfoximine is suitable for use to:
- examine whether the inhibition of glutathione by BSO enhances the apoptotic effect of estrogen on antihormone-resistant human breast cancer cells
- investigate the effect of BSO on development of bovine embryos
- inhibit GSH in several studies
- investigate the effect of GSH synthesis on oocyte maturation
생화학적/생리학적 작용
DL-Buthionine-sulfoximine inhibits the biosynthesis of Glutathione (GSH) in liver and other peripheral organs. It does not have any effect on GSH in the CNS. It augments the antiproliferative action of reactive oxygen species (e.g., hydrogen peroxide), and agents that indirectly cause accumulation of reactive oxygen species (e.g., 2-methoxyestradiol, which increases intracellular superoxide anion).
기타 정보
Depletes glutathionine in isolated cells
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Evaluation of hepatotoxicity and nephrotoxicity of natural food colorants in mice depleted of glutathione by DL-buthionine sulfoximine.
Kawazoe S, et al.
Journal of Health Science, 46, 56-58 (2000)
Joan S Lewis-Wambi et al.
Breast cancer research : BCR, 10(6), R104-R104 (2008-12-09)
Estrogen deprivation using aromatase inhibitors is one of the standard treatments for postmenopausal women with estrogen receptor (ER)-positive breast cancer. However, one of the consequences of prolonged estrogen suppression is acquired drug resistance. Our group is interested in studying antihormone
Yuka Hirai et al.
Biological & pharmaceutical bulletin, 29(5), 1064-1067 (2006-05-03)
2-Methoxyestradiol (2-ME), an endogenous metabolite of 17beta-estradiol, induces the intracellular accumulation of superoxide anion (O2*-) and buthionine sulfoximine (BSO) is an inhibitor of glutathione (GSH) synthesis. We have examined the combination anticancer effect of 2-ME and BSO accompanied with hydrogen
M A Baker et al.
Analytical biochemistry, 190(2), 360-365 (1990-11-01)
By combining the least complicated and expedient methods of sample handling with the sensitivity and specificity of the GSH assay by enzymatic recycling and the small volumes and software capabilities of microtiter plate technology we have devised a rapid, sensitive
M Takahashi et al.
Biology of reproduction, 49(2), 228-232 (1993-08-01)
The purpose of this investigation was to determine the effect of beta-mercaptoethanol (beta-ME) and cysteamine, low-molecular-weight thiol compounds, on the development and intracellular glutathione content of bovine embryos obtained by in vitro fertilization of in vitro-matured oocytes. Embryos developed to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.