추천 제품
vapor density
3.9 (vs air)
Quality Level
vapor pressure
97.5 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
99%
양식
liquid
불순물
≤0.05% water
refractive index
n20/D 1.3 (lit.)
pH
1 (10 g/L)
bp
72.4 °C (lit.)
mp
−15.4 °C (lit.)
solubility
ethanol: soluble 0.33 mL/mL
density
1.489 g/mL at 20 °C (lit.)
SMILES string
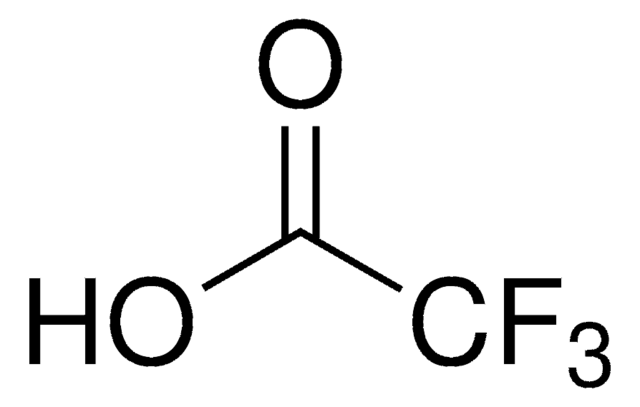
OC(C(F)(F)F)=O
InChI
1S/C2HF3O2/c3-2(4,5)1(6)7/h(H,6,7)
InChI key
DTQVDTLACAAQTR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Trifluoroacetic acid (TFA) is an organofluorine compound used as a reagent in organic synthesis for various acid-catalyzed reactions such as ring-opening of epoxides, biomimetic cyclization, Cope rearrangements, and natural product synthesis. TFA′s physicochemical characteristics provide advantages over other acids because of its high volatility, solubility in organic solvents, and acidic strength. When TFA is used as a reagent the product isolation is simple by evaporation due to its very high volatility. Less volatile acids such as sulfuric acid or p-toluenesulfonic acid may require neutralization or an extractive workup.
애플리케이션
Trifluoroacetic acid can be used as a reagent:
TFA can also be used as:
- For the cleavage of nitrogen and oxygen protecting groups such as N-Boc, N-benzyloxymethyl, benzyl ether, p-methoxybenzyl ether, t-butyl ether, t-butyloxymethyl ether, triphenylmethyl ether, and dimethyl acetals.
- In the Baeyer–Villiger oxidation reactions in combination with sodium percarbonate.,·
- For the C-H trifluoromethylation of arenes.
TFA can also be used as:
- A solvent in atom transfer cyclization reactions and polymer processes.
- A catalyst in the synthesis of ε-caprolactam via Beckmann rearrangement of cyclohexanone oxime in aprotic solvents.
포장
1mL in each ampule.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
이미 열람한 고객
Organocatalyzed Beckmann rearrangement of cyclohexanone oxime by trifluoroacetic acid in aprotic solvent
Ronchin L, et al.
Catalysis Communications, 10(2), 251-256 (2008)
Guillaume Plourde et al.
Lancet (London, England), 386(10009), 2192-2203 (2015-09-29)
Transradial access for cardiac catheterisation results in lower bleeding and vascular complications than the traditional transfemoral access route. However, the increased radiation exposure potentially associated with transradial access is a possible drawback of this method. Whether transradial access is associated
Yukiko Enomoto-Rogers et al.
Carbohydrate polymers, 92(2), 1827-1834 (2013-02-13)
Fully substituted glucomannan triacetate (GMTAc) (degree of substitution (DS)=3.0) was prepared from konjac glucomannan (KGM) treated with acetic acid and trifluoroacetic anhydride (TFAA). The peaks in the (1)H- and (13)C NMR spectra of GMTAc were assigned in detail based on
Caroline Le Maréchal et al.
Veterinary research, 42, 35-35 (2011-02-18)
Staphylococcus aureus is a major cause of mastitis in ruminants. In ewe mastitis, symptoms range from subclinical to gangrenous mastitis. S. aureus factors or host-factors contributing to the different outcomes are not completely elucidated. In this study, experimental mastitis was
Mian Qi et al.
Journal of the American Chemical Society, 136(43), 15366-15378 (2014-10-18)
Distance measurement in the nanometer range by electron paramagnetic resonance spectroscopy (EPR) in combination with site-directed spin labeling is a very powerful tool to monitor the structure and dynamics of biomacromolecules in their natural environment. However, in-cell application is hampered
프로토콜
Fmoc resin cleavage and deprotection follows the difficult task of detaching the peptide from the resin support and removing all the side-chain protecting groups of the amino acid residues to yield the desired peptide.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.