모든 사진(1)
About This Item
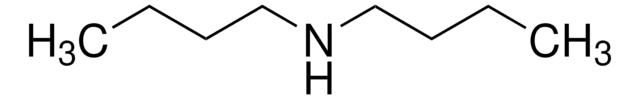
Linear Formula:
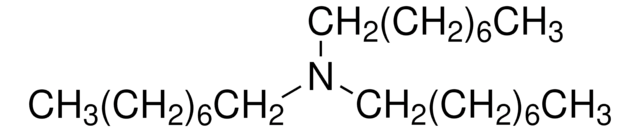
[CH3(CH2)3]3N
CAS Number:
Molecular Weight:
185.35
Beilstein:
1698872
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.21
bp:
216 °C (lit.)
vapor pressure:
0.3 mmHg ( 20 °C)
2.4 mmHg ( 55 °C)
2.4 mmHg ( 55 °C)
추천 제품
vapor density
6.38 (vs air)
Quality Level
vapor pressure
0.3 mmHg ( 20 °C)
2.4 mmHg ( 55 °C)
분석
≥98.5%
양식
liquid
autoignition temp.
410 °F
expl. lim.
6 %
refractive index
n20/D 1.428 (lit.)
pH
10.2 (25 °C, 0.1 g/L)
bp
216 °C (lit.)
mp
−70 °C (lit.)
density
0.778 g/mL at 25 °C (lit.)
SMILES string
CCCCN(CCCC)CCCC
InChI
1S/C12H27N/c1-4-7-10-13(11-8-5-2)12-9-6-3/h4-12H2,1-3H3
InChI key
IMFACGCPASFAPR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Tributylamine (TBA) is a tertiary amine used as strong base anion (SBA) exchange resin. TBA can also be used as a corrosion inhibitor for mild steel in HCl solution. Additionally, it can be used as a base solvent in various organic synthesis.
애플리케이션
Tributylamine may be used as:
- An extraction solvent with CHCA (α-cyano-4-hydroxycinnamic acid) for the selective phospholipids (PLs) extraction from EVOO (extra virgin olive oil) and HO (hazelnut oil).
- A hydroxylating agent in the synthesis of spinel nickel ferrites (NiFe2O4) nanoparticles (NPs).
특징 및 장점
In various co-precipitation methods, tributylamine is used as a hydroxylating agent instead of NaOH and NH4OH since it causes a uniform rise in pH which prevents local supersaturation and promotes homogeneous nucleation throughout the solution.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point (°F)
145.4 °F - closed cup
Flash Point (°C)
63 °C - closed cup
이미 열람한 고객
Martin Enmark et al.
Analytical and bioanalytical chemistry, 411(15), 3383-3394 (2019-04-26)
This study presents a systematic investigation of factors influencing the chromatographic separation of diastereomers of phosphorothioated pentameric oligonucleotides as model solutes. Separation was carried out under ion-pairing conditions using an XBridge C18 column. For oligonucleotides with a single sulfur substitution
N Ghoneim
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 57(3), 483-489 (2001-04-13)
The influence of temperature on the efficiency of fluorescence of the encounter complex (A- ...D+)* in intermolecular electron transfer starting from neutral precursors has been studied. In contrast to what is generally assumed, fluorescence emission of the exciplexes is found
Steven A Steiner et al.
Journal of chromatography. A, 1192(1), 152-156 (2008-04-15)
A background electrolyte for capillary electrophoresis containing tris(-hydroxymethyl) aminomethane (THAM) and ethanesulfonic acid (ESA) gives excellent efficiency for separation of drug cations with actual theoretical plate numbers as high as 300,000. However, the analyte cations often elute too quickly and
A Touati et al.
Journal of protein chemistry, 11(6), 613-621 (1992-12-01)
The study of the solubility of unstructured proteins (alpha s1-, beta-, and kappa-casein) and well-structured globulin (beta-lactoglobulin) in low water binary solvent systems demonstrated the crucial importance of solvent polarity and neutralization of protein polar functions on the final outcome
Alexander S Misharin et al.
Analytical chemistry, 77(2), 459-470 (2005-01-15)
The analytical performance of an atmospheric pressure sampling, multiple-channel, high-throughput mass spectrometer was investigated using samples of a variety of types. The instrument, based on an array of cylindrical ion traps, was built with four independent channels and here is
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.