모든 사진(3)
About This Item
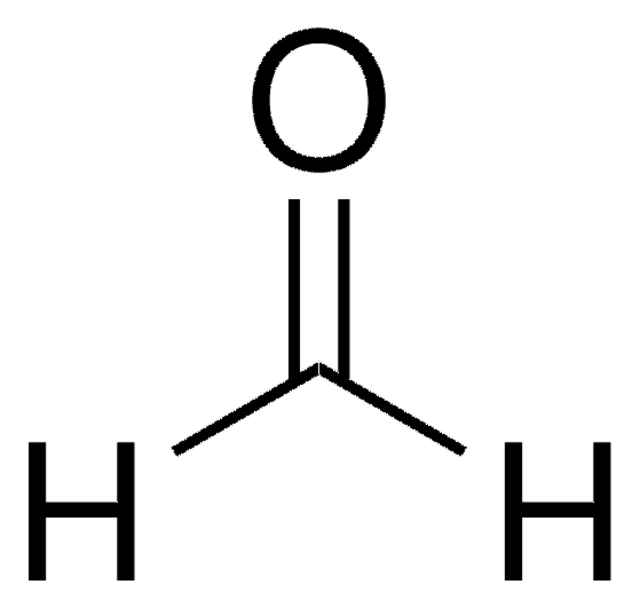
실험식(Hill 표기법):
CH3NO2
CAS Number:
Molecular Weight:
61.04
Beilstein:
1698205
EC Number:
MDL number:
UNSPSC 코드:
12190000
PubChem Substance ID:
NACRES:
NA.21
bp:
101.2 °C (lit.)
vapor pressure:
2.7 mmHg
추천 제품
Grade
HPLC grade
Quality Level
vapor density
2.1 (vs air)
vapor pressure
2.7 mmHg
분석
≥96%
양식
liquid
autoignition temp.
784 °F
expl. lim.
7.3 %, 33 °F
기술
HPLC: suitable
불순물
<0.030% water
refractive index
n20/D 1.382 (lit.)
pH
6.4 (20 °C, 0.01 g/L)
bp
101.2 °C (lit.)
mp
−29 °C (lit.)
density
1.127 g/mL at 25 °C (lit.)
λ
H2O reference
UV 흡수
λ: 380 nm Amax: 1.00
λ: 386 nm Amax: 0.50
λ: 395 nm Amax: 0.20
λ: 400 nm Amax: 0.10
λ: 405 nm Amax: 0.05
λ: 430-700 nm Amax: 0.01
응용 분야
food and beverages
SMILES string
C[N+]([O-])=O
InChI
1S/CH3NO2/c1-2(3)4/h1H3
InChI key
LYGJENNIWJXYER-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Asymmetric aza-Henry reaction toward trifluoromethyl β-nitroamines and biological investigation of their adamantane-type derivatives.: This study used nitromethane in asymmetric aza-Henry reactions to synthesize trifluoromethyl β-nitroamines, which were further investigated for their biological properties, showcasing the potential of nitromethane in advanced synthetic chemistry (Ren et al., 2024).
- Effect of Temperature on the Liquid Bridging Force while Maintaining Physical Stability in Solid-Liquid Mixed Fuel.: Nitromethane was analyzed in this research to understand its role in the stability of solid-liquid mixed fuels under varying temperatures, providing insights into the optimization of fuel formulations (Zhang et al., 2024).
- Generation of New Synthons for Synthesis Through Activation of Nitromethane.: This research demonstrated the activation of nitromethane to generate new synthons for synthetic applications, highlighting its versatility and importance in creating novel chemical entities (Wang et al., 2024).
- Towards Chemoenzymatic Syntheses of Both Enantiomers of Phosphoemeriamine.: The study explored the use of nitromethane in chemoenzymatic syntheses, enabling the production of both enantiomers of phosphoemeriamine, an important compound in chemical biology (Kiełbasiński et al., 2024).
- Rationally introducing non-canonical amino acids to enhance catalytic activity of LmrR for Henry reaction.: Nitromethane was employed in this study to investigate the enhancement of catalytic activity in the Henry reaction through the introduction of non-canonical amino acids, demonstrating its significance in enzyme catalysis research (Wang et al., 2024).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 3 - Repr. 2
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Flash Point (°F)
95.0 °F - closed cup
Flash Point (°C)
35 °C - closed cup
The catalytic chemistry of nitromethane over Co-ZSM5 and other catalysts in connection with the methane-NOxSCR reaction.
Cowan AD, et al.
J. Catal., 176(2), 329-343 (1998)
Metal ion encapsulation: cobalt cages derived from polyamines, formaldehyde, and nitromethane.
Geue RJ, et al.
Journal of the American Chemical Society, 106(19), 5478-5488 (1984)
Benedek Vakulya et al.
Organic letters, 7(10), 1967-1969 (2005-05-07)
Cinchona alkaloid-derived chiral bifunctional thiourea organocatalysts were synthesized and applied in the Michael addition between nitromethane and chalcones with high ee and chemical yields.
Atanu Bhattacharya et al.
The Journal of chemical physics, 136(2), 024321-024321 (2012-01-21)
Decomposition of electronically excited nitro-containing molecules with different X-NO(2) (X = C, N, O) moieties has been intensively investigated over the past decades; however, their decomposition behavior has not previously been compared and contrasted. Comparison of their unimolecular decomposition behavior
Eagleson M.
Concise Encyclopedia Chemistry, 696-696 (1994)
프로토콜
GC Analysis of Class 2 Residual Solvents on OVI-G43
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.