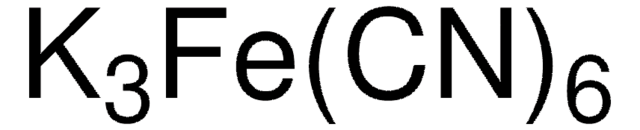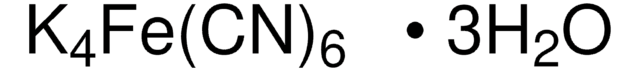추천 제품
Quality Level
분석
≥97.0%
양식
crystalline
반응 적합성
reagent type: catalyst
core: cobalt
불순물
≤0.1% free cyanide
density
1.878 g/mL at 25 °C (lit.)
음이온 미량물
sulfate (SO42-): ≤500 mg/kg
양이온 미량물
Fe: ≤50 mg/kg
적합성
suitable for acidity or alkalinity (alkalinity <= 0.15 meq/g)
SMILES string
[K+].[K+].[K+].N#C[Co-3](C#N)(C#N)(C#N)(C#N)C#N
InChI
1S/6CN.Co.3K/c6*1-2;;;;/q;;;;;;-3;3*+1
InChI key
VSUFNKULKBVQQW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Potassiumhexacyanocobaltate(III) can be used as a complexing agent to synthesize double metal cyanide catalyst:
- For the chemoselective reductive amination of carbonyl compounds with aromatic amines to synthesize substituted amines in the presence of polymethylhydrosiloxane as a reducing agent.
- In the ring opening polymerization of propylene oxide to synthesize polyols.
- In the coupling reaction of CO2 with hydrous epoxides to synthesize cyclic carbonates.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Edward E Little et al.
Environmental science and pollution research international, 14(5), 333-337 (2007-08-29)
Cobalt cyanide complexes often result when ore is treated with cyanide solutions to extract gold and other metals. These have recently been discovered in low but significant concentrations in effluents from gold leach operations. This study was conducted to determine
A 59Co NMR relaxation probe of macromolecule anionic binding sites.
T Raj et al.
Analytical biochemistry, 106(2), 373-376 (1980-08-01)
Reynhardt EC and Boeyens JCA.
Acta Crystallographica Section B, Structural Science, Crystal Engineering and Materials, 28(2), 524-529 (1972)
Effect of pH on Retention of 134Cs and 60Co from MAW by Potassium Hexacyanocobaltate(III).
Mekhail FM, et al.
Isotopes in Environmental and Health Studies, 130-133 (1991)
Xin Zhang et al.
ChemSusChem, 14(1), 467-478 (2020-10-13)
Electrolytic water splitting using surplus electricity represents one of the most cost-effective and promising strategies for hydrogen production. The high overpotential of the oxygen-evolution reaction (OER) caused by the multi-electron transfer process with a high chemical energy barrier, however, limits
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.