모든 사진(2)
About This Item
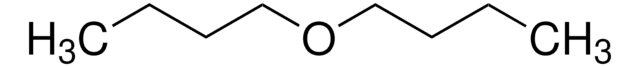
Linear Formula:
[CH3(CH2)3]2O
CAS Number:
Molecular Weight:
130.23
Beilstein:
1732752
EC Number:
MDL number:
UNSPSC 코드:
12352112
PubChem Substance ID:
NACRES:
NA.21
bp:
142-143 °C (lit.)
vapor pressure:
4.8 mmHg ( 20 °C)
추천 제품
vapor density
4.48 (vs air)
Quality Level
vapor pressure
4.8 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
≥99%
양식
liquid
autoignition temp.
365 °F
expl. lim.
8.5 %
dilution
(for general lab use)
refractive index
n20/D 1.399 (lit.)
pH
5.2
bp
142-143 °C (lit.)
mp
−98 °C (lit.)
density
0.764 g/mL at 25 °C (lit.)
SMILES string
CCCCOCCCC
InChI
1S/C8H18O/c1-3-5-7-9-8-6-4-2/h3-8H2,1-2H3
InChI key
DURPTKYDGMDSBL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Dibutyl ether is commonly used as a solvent for various reactions. It forms azeotropic compositions with various organic solvents and the vapor/liquid equilibria of its binary mixtures have been investigated by head-space gas chromatography.
애플리케이션
Dibutyl ether may be used to compose the solvent mixtures in the following studies:
- To evaluate the anthracene solubility in various binary solvent mixtures composed of dibutyl ether along with n-hexane, cyclohexane, n-heptane, methylcyclohexane, n-octane, isooctane and cyclooctane.
- To measure the pyrene solubility in various binary solvent mixtures composed of dibutyl ether along with n-hexane, cyclohexane, n-heptane, methylcyclohexane, n-octane, isooctane and tert-butylcyclohexane.
- To investigate the reaction mechanism and kinetic studies of the reaction of phenyl isocyanate with methanol in the presence of dibutyltin diacetate (catalyst) to afford urethane. Rate constant of the reaction, k was reported to be 0.96liter/(mole sec).
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
82.4 °F - closed cup
Flash Point (°C)
28 °C - closed cup
Natasha A Andrade et al.
Environmental pollution (Barking, Essex : 1987), 222, 412-422 (2017-01-21)
Polybrominated diphenyl ethers (PBDEs) may enter the environment because of accumulation in biosolids followed by application to agricultural lands. No published dissipation studies are available for PBDEs in agricultural soils after biosolids application. Therefore, we conducted a 3-year study to
Solubility of pyrene in binary solvent mixtures containing dibutyl ether.
Wallach JR, et al.
Journal of Chemical and Engineering Data, 34(1), 70-73 (1989)
Zhao Chen et al.
Electrophoresis, 38(22-23), 3036-3047 (2017-07-18)
In the present study, a monolithic capillary column with higher permeability was developed for the in vivo discrimination of four coumarin analogs (bergapten, 2'-acetylangelicin, imperatorin, and osthole) that typically require long separation times in HPLC. Instead of conventional methacrylate ester
Kinetics and mechanism of urethane formation catalyzed by organotin compounds. I. The reaction of phenyl isocyanate with methanol in dibutyl ether under the action of dibutyltin diacetate.
Van der Weij FW.
Journal of Polymer Science Part A: Polymer Chemistry, 19(2), 381-388 (1981)
Solubility of anthracene in binary solvent mixtures containing dibutyl ether.
Marthandan MV and Acree Jr WE.
Journal of Chemical and Engineering Data, 32(3), 301-303 (1987)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.