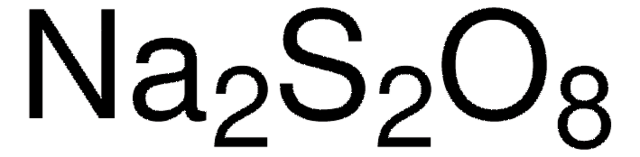모든 사진(3)
About This Item
Linear Formula:
Na2S2O8
CAS Number:
Molecular Weight:
238.10
EC Number:
MDL number:
UNSPSC 코드:
12161700
PubChem Substance ID:
NACRES:
NA.25
solubility:
H2O: 1 M at 20 °C, clear, colorless
추천 제품
제품 라인
BioXtra
Quality Level
분석
≥99%
반응 적합성
reagent type: oxidant
불순물
<0.0005% Phosphorus (P)
<0.1% Insoluble matter
solubility
H2O: 1 M at 20 °C, clear, colorless
음이온 미량물
chloride (Cl-): <0.05%
양이온 미량물
Al: <0.0005%
Ca: <0.005%
Cu: <0.0005%
Fe: <0.0005%
K: <0.02%
Mg: <0.001%
Pb: <0.001%
Zn: <0.0005%
SMILES string
[Na+].[Na+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2Na.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
InChI key
CHQMHPLRPQMAMX-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
<ul>
<li><strong>Electrochemical treatment of organic pollutants in landfill leachate using a three-dimensional electrode system.</strong>: This study explores the electrochemical treatment of landfill leachate using a three-dimensional electrode system. Sodium persulfate is used as an oxidizing agent to degrade organic pollutants effectively, providing a potential method for waste management and environmental protection (Yu et al., 2020).</li>
<li><strong>The Box-Benkhen experimental design for the optimization of the electrocatalytic treatment of wastewaters with high concentrations of phenol and organic matter.</strong>: This paper discusses the optimization of electrocatalytic treatment processes for wastewater containing high levels of phenol and organic matter using sodium persulfate. The study provides valuable insights for improving wastewater treatment efficiency (GilPavas et al., 2009).</li>
<li><strong>Reaction of pectin and glycidyl methacrylate and ulterior formation of free films by reticulation.</strong>: This research involves the chemical modification of pectin with glycidyl methacrylate followed by cross-linking using sodium persulfate, leading to the formation of free-standing films. These films have potential applications in pharmaceuticals and food packaging (Maior et al., 2008).</li>
</ul>
<li><strong>Electrochemical treatment of organic pollutants in landfill leachate using a three-dimensional electrode system.</strong>: This study explores the electrochemical treatment of landfill leachate using a three-dimensional electrode system. Sodium persulfate is used as an oxidizing agent to degrade organic pollutants effectively, providing a potential method for waste management and environmental protection (Yu et al., 2020).</li>
<li><strong>The Box-Benkhen experimental design for the optimization of the electrocatalytic treatment of wastewaters with high concentrations of phenol and organic matter.</strong>: This paper discusses the optimization of electrocatalytic treatment processes for wastewater containing high levels of phenol and organic matter using sodium persulfate. The study provides valuable insights for improving wastewater treatment efficiency (GilPavas et al., 2009).</li>
<li><strong>Reaction of pectin and glycidyl methacrylate and ulterior formation of free films by reticulation.</strong>: This research involves the chemical modification of pectin with glycidyl methacrylate followed by cross-linking using sodium persulfate, leading to the formation of free-standing films. These films have potential applications in pharmaceuticals and food packaging (Maior et al., 2008).</li>
</ul>
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
N Sabri et al.
The Science of the total environment, 427-428, 382-389 (2012-05-15)
The objective of this work was to evaluate the removal of ibuprofen (IBP) using the oxidants hydrogen peroxide (H(2)O(2)) and sodium persulfate (Na(2)S(2)O(8)). The ability of magnetite (Fe(3)O(4)) to activate persulfate (PS) and H(2)O(2) for the oxidation of IBP at
Mushtaque Ahmad et al.
Journal of contaminant hydrology, 115(1-4), 34-45 (2010-05-05)
Persulfate dynamics in the presence of subsurface minerals was investigated as a basis for understanding persulfate activation for in situ chemical oxidation (ISCO). The mineral-mediated decomposition of persulfate and generation of oxidants and reductants was investigated with four iron and
Aikaterini Tsitonaki et al.
Water research, 42(4-5), 1013-1022 (2007-10-19)
The effects of heat-activated persulfate on indigenous microorganisms and microcosms augmented with Pseudomonas putida KT2440 were studied in laboratory batch reactors with aquifer material. Microscopic enumeration was used to measure the changes in cell density, and acetate consumption was used
Richard L Johnson et al.
Environmental science & technology, 42(24), 9350-9356 (2009-01-30)
Contaminant destruction with in situ chemical oxidation (ISCO) using persulfate (peroxydisulfate, S2O8(2-)) can be enhanced by activation, which increases the rate of persulfate decomposition to sulfate radicals (SO4*-). This step initiates a chain of radical reactions involving species (including SO4*-
Chenju Liang et al.
Water research, 42(15), 4091-4100 (2008-08-23)
The present study focused on evaluation of activated persulfate (PS) anion (S(2)O(8)(2-)) oxidative degradation of benzene, toluene, ethylbenzene, and xylene (constituents of gasoline and known collectively as BTEX) contamination. The results indicated that BTEX were effectively oxidized by PS in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.