추천 제품
Grade
pharmaceutical primary standard
API family
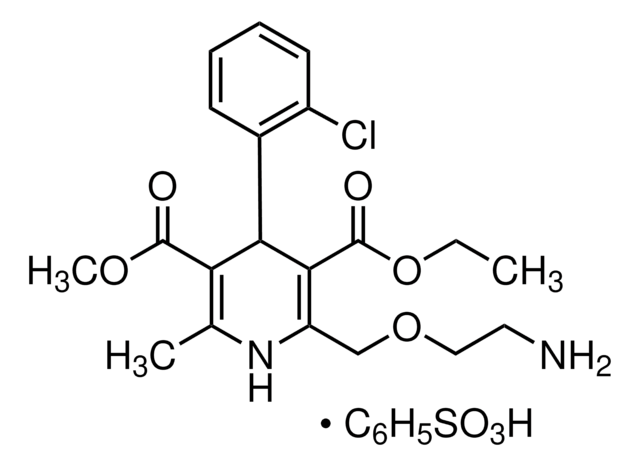
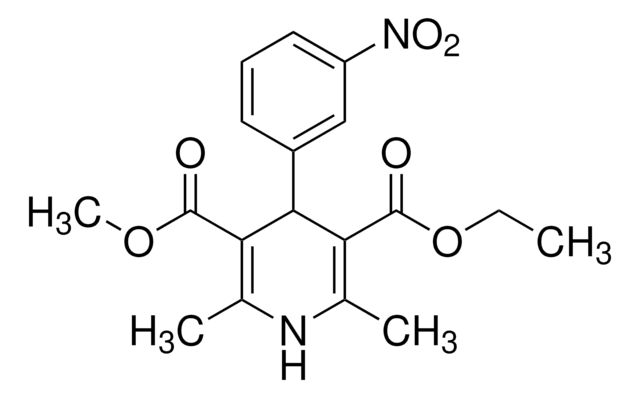
nitrendipine
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
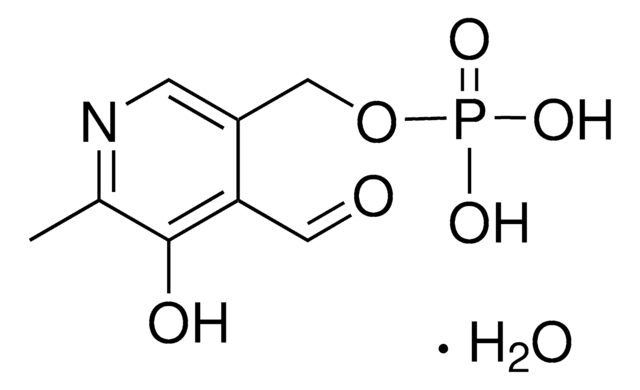
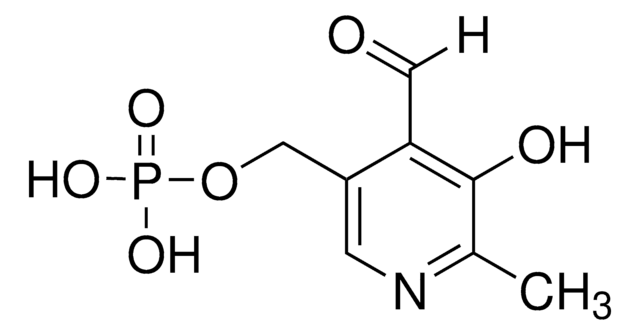
SMILES string
[N+](=O)([O-])c1cc(ccc1)c2c(c(nc(c2C(=O)OC)C)C)C(=O)OCC
InChI
1S/C18H18N2O6/c1-5-26-18(22)15-11(3)19-10(2)14(17(21)25-4)16(15)12-7-6-8-13(9-12)20(23)24/h6-9H,5H2,1-4H3
InChI key
YEHVABIPGJLMET-UHFFFAOYSA-N
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Nitrendipine impurity A EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
가장 최신 버전 중 하나를 선택하세요:
G R Aronoff
Journal of cardiovascular pharmacology, 6 Suppl 7, S974-S976 (1984-01-01)
To determine the effect of decreased renal function on the elimination kinetics of the calcium entry blocker nitrendipine and its metabolite, we gave 18 subjects with various levels of renal dysfunction a single 20-mg oral dose of nitrendipine. Plasma nitrendipine
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.