B5274
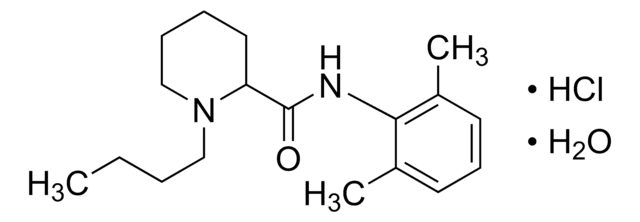
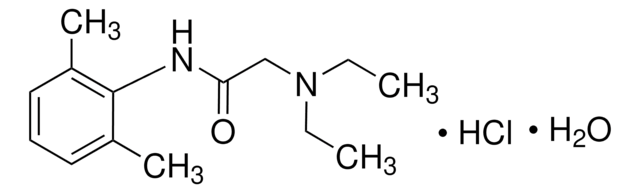
Bupivacaine hydrochloride monohydrate
analytical standard, for drug analysis
동의어(들):
1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C18H28N2O · HCl · H2O
CAS Number:
Molecular Weight:
342.90
EC Number:
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Quality Level
분석
≥99%
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
forensics and toxicology
pharmaceutical (small molecule)
veterinary
형식
neat
SMILES string
O.Cl.CCCCN1CCCCC1C(=O)Nc2c(C)cccc2C
InChI
1S/C18H28N2O.ClH.H2O/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3;;/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21);1H;1H2
InChI key
HUCIWBPMHXGLFM-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Bupivacaine hydrochloride monohydrate may be used as an analytical standard for the determination of the bupivacaine in pharmaceutical preparations and plasma samples using chromatography techniques.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
생화학적/생리학적 작용
Sodium channel blocker, local anesthetic.
애플리케이션
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 2 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Determination of free concentration of ropivacaine and bupivacaine in blood plasma by ultrafiltration and coupled-column liquid chromatography
Arvidsson T and Eklund E
Journal of Chromatography. B, Biomedical Sciences and Applications, 668(1), 91-98 (1995)
Anna M Wiersma et al.
Bio-protocol, 8(10), e2862-e2862 (2018-05-20)
When injected into the motor cortex of rats, anterograde tracers label fibers of the associated descending corticospinal tract (CST) that originate from pyramidal neurons in the tracer-injected cortex. These fibers can be assessed at the level of the spinal cord
Kejian Shi et al.
Anesthesia and analgesia, 116(4), 804-809 (2013-03-06)
While lipid emulsion may reverse the systemic toxicity of bupivacaine, the pharmacokinetics and tissue distribution of bupivacaine after lipid emulsion infusion are not clear. In this study, we assessed the influence of lipid emulsion administration on the pharmacokinetics and tissue
Brian M Ilfeld et al.
Anesthesia and analgesia, 117(5), 1248-1256 (2013-10-11)
Currently available local anesthetics approved for single-injection peripheral nerve blocks have a maximum duration of <24 hours. A liposomal bupivacaine formulation (EXPAREL, Pacira Pharmaceuticals, Inc., San Diego, CA), releasing bupivacaine over 96 hours, recently gained Food and Drug Administration approval
Ilin Kuo et al.
Anesthesiology, 118(6), 1350-1361 (2013-03-06)
In vitro observations support the lipid sink theory of therapeutic action by confirming the capacity of lipid emulsions to successfully uptake bupivacaine from aqueous media. However, competing hypotheses and some in/ex vivo small animal studies suggest that a metabolic or
Chromatograms
application for HPLCsuitable for GCapplication for HPLCapplication for HPLC자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.