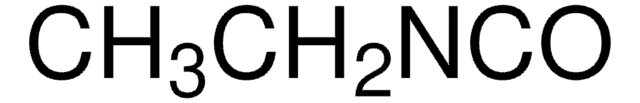91095
Trichloroacetyl isocyanate
purum, ≥97.0% (GC)
동의어(들):
2,2,2-Trichloroacetyl isocyanate, alpha,alpha,alpha-Trichloroacetyl isocyanate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
Cl3CCONCO
CAS Number:
Molecular Weight:
188.40
Beilstein:
971201
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
grade
purum
Quality Level
분석
≥97.0% (GC)
양식
liquid
refractive index
n20/D 1.480 (lit.)
n20/D 1.480
bp
80-85 °C/20 mmHg (lit.)
density
1.581 g/mL at 25 °C (lit.)
작용기
amine
chloro
isocyanate
저장 온도
2-8°C
SMILES string
ClC(Cl)(Cl)C(=O)N=C=O
InChI
1S/C3Cl3NO2/c4-3(5,6)2(9)7-1-8
InChI key
GRNOZCCBOFGDCL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Trichloroacetyl isocyanate (TAI) is a versatile building block in organic synthesis. It is also used as an in situ derivatizing reagent for the characterization of phenols, alcohols, and amines.
애플리케이션
- One-pot synthesis of 2-acylaminobenzimidazoles: A study on the synthesis of 2-acylaminobenzimidazoles via a reaction between trichloroacetyl isocyanate and 1,2-phenylenediamine derivatives (Shajari et al., 2018).
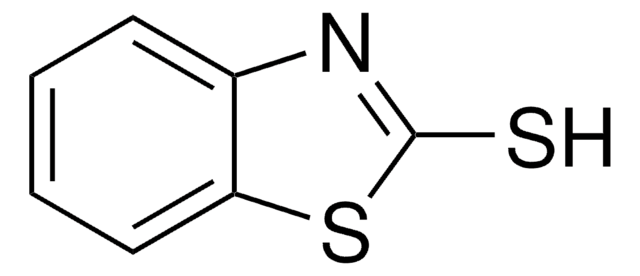
- Cabamothioate compounds: Research on the synthesis of S-aryl (trichloroacetyl) carbamothioate from a reaction of 2-naphthalenethiol or thiophenol derivatives and trichloroacetyl isocyanate (Shajari et al., 2021).
- Chiral calyx[4]arenes: Diastereoselective synthesis of inherently chiral calyx[4]arenes via reaction of trichloroacetyl isocyanate with 1,3-dihydroxy calixarene (Boyko et al., 2016).
기타 정보
Derivatizing reagent for the structural assignment to hydroxy compounds by NMR; Used in negative ion chemical ionization mass spectrometry
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
150.8 °F - closed cup
Flash Point (°C)
66 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
D.R. Taylor
Canadian Journal of Chemistry, 54, 189-189 (1976)
M. Budesinsky et al.
Collection of Czechoslovak Chemical Communications, 45, 2784-2784 (1980)
Determination of chain branching in epoxy resins by nuclear magnetic resonance spectrometry.
H D Mak et al.
Analytical chemistry, 44(4), 837-839 (1972-04-01)
H. Fujiwara et al.
Pract. Spectrosc., 3, 329-329 (1980)
Ian Paterson et al.
Angewandte Chemie (International ed. in English), 53(10), 2692-2695 (2014-02-01)
Leiodermatolide is an antimitotic macrolide isolated from the marine sponge Leiodermatium sp. whose potentially novel tubulin-targeting mechanism of action makes it an exciting lead for anticancer drug discovery. In pursuit of a sustainable supply, we report a highly stereocontrolled total
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.