추천 제품
grade
purum
Quality Level
분석
≥96.5% (HPLC)
양식
powder
mp
119-122 °C (lit.)
120-124 °C
작용기
ketone
SMILES string
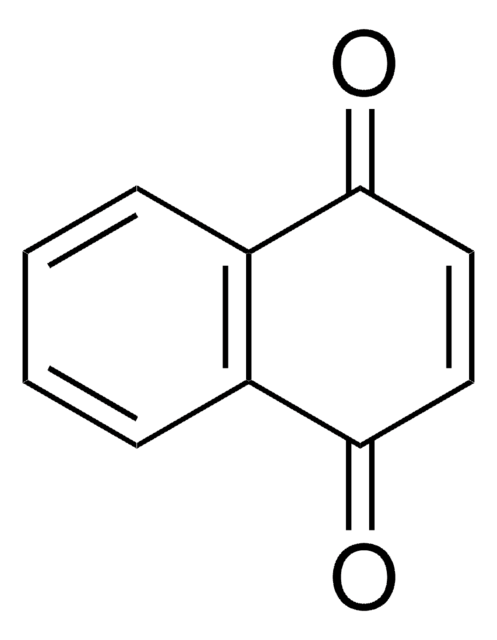
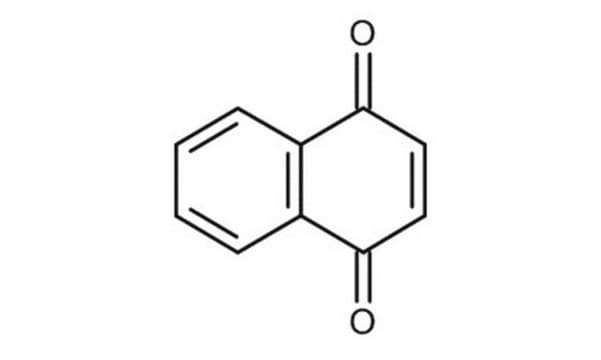
O=C1C=CC(=O)c2ccccc12
InChI
1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H
InChI key
FRASJONUBLZVQX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
1,4-Naphthoquinone is the key structural moiety of many anticancer and antifungal agents.
It can be used to synthesize:
Additional appilcation include:
It can be used to synthesize:
- 3,3-Disubstituted oxindoles via asymmetric Michael addition to oxindole.
- Bioactive isoindolines via asymmetric 1,3-dipolar cycloaddition to azomethine ylides generated in situ from aldehydes and diethyl aminomalonate.
- α,α-Difluoro-β-hydroxy ketone via ‘on water′ catalyst-free Mukaiyama-aldol reaction with difluoroenoxysilane.
- 2-Hydroxy-3-anilino-1,4-naphthoquinone, which shows potent in vivo antimalarial activity.
Additional appilcation include:
- As an arylation reagent for the α-arylation of aldehydes.
- As a starting material in the multi-step synthesis of benz[f]indole-4,9-diones.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
285.8 °F
Flash Point (°C)
141 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
1, 4-Naphthoquinone.
Yu J S.
Synlett, 25(16), 2377-2378 (2014)
Design, synthesis and evaluation of novel 1, 4-naphthoquinone derivatives as antifungal and anticancer agents.
Tandon V K, et al.
Bioorganic & Medicinal Chemistry Letters, 14(5), 1079-1083 (2004)
Rita Gatti et al.
Analytical and bioanalytical chemistry, 405(2-3), 817-825 (2012-07-24)
The use of 1,4-naphthoquinone as an advantageous pre-column reagent for liquid chromatography analysis of aliphatic thiol compounds is proposed. The compound reacts selectively in mild conditions (5 min at room temperature; pH 7.5) with thiol function. The resulting adducts were
Paula F Carneiro et al.
Bioorganic & medicinal chemistry, 20(16), 4995-5000 (2012-07-17)
New oxirane derivatives were synthesized using six naphthoquinones as the starting materials. Our biological results showed that these oxiranes acted as trypanocidal agents against Trypanosoma cruzi with minimal cytotoxicity in the VERO cell line compared to naphthoquinones. In particular, oxirane
Yu Shang et al.
Environmental science & technology, 46(5), 2935-2942 (2012-02-01)
Airborne quinones contribute to adverse health effects of ambient particles probably because of their ability to generate hydroxyl radicals (·OH) via redox cycling, but the mechanisms remain unclear. We examined the chemical mechanisms through which 1,4-naphthoquinone (1,4-NQ) induced ·OH, and
프로토콜
US EPA Method 8270 (Appendix IX): GC Analysis of Semivolatiles on Equity®-5 (30 m x 0.25 mm I.D., 0.50 μm)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.