67770
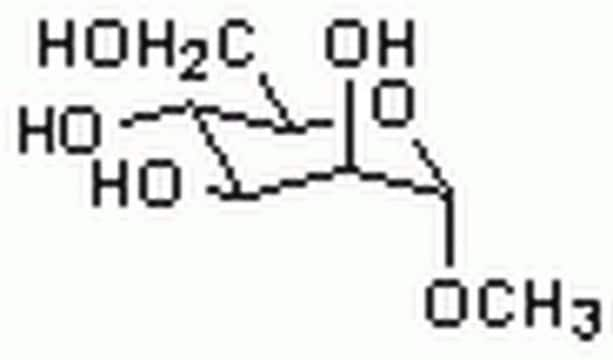
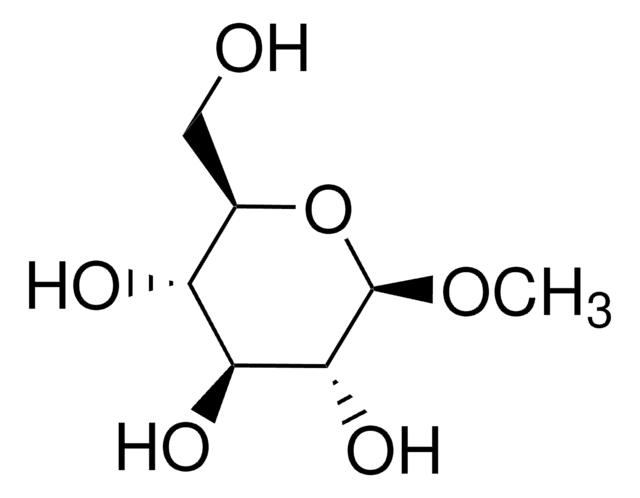
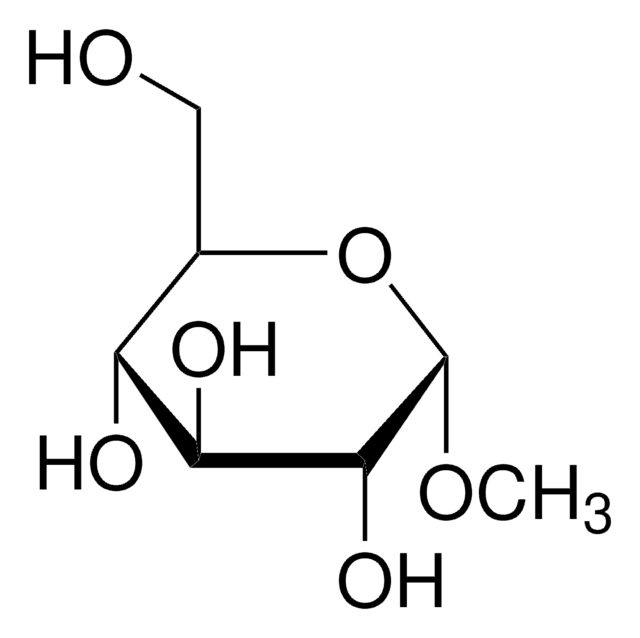
Methyl α-D-mannopyranoside
≥99.0%, suitable for microbiology, enables differentiation between species of Listeria
동의어(들):
Methyl alpha-D-mannoside, Methyl-alpha-D-mannopyranoside, α-Methyl D-mannoside
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C7H14O6
CAS Number:
Molecular Weight:
194.18
Beilstein:
81566
EC Number:
MDL number:
UNSPSC 코드:
41106212
PubChem Substance ID:
NACRES:
NA.85
추천 제품
Quality Level
분석
≥99.0% (sum of enantiomers, HPLC)
≥99.0%
양식
powder
광학 활성
[α]20/D 77.0 to 82.0°, c = 10% in H2O
분자량
194.18 g/mol
mp
187-195 °C
193-196 °C (lit.)
solubility
H2O: 0.1 g/mL, clear, colorless
응용 분야
microbiology
SMILES string
CO[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O
InChI
1S/C7H14O6/c1-12-7-6(11)5(10)4(9)3(2-8)13-7/h3-11H,2H2,1H3/t3-,4-,5+,6+,7+/m1/s1
InChI key
HOVAGTYPODGVJG-VEIUFWFVSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Methyl α-D-mannopyranoside is a compound that belongs to the class of organic compounds known as o-glycosyl compounds. It is used for the differentiation of Listeria species, Listeria monocytogenes, Listeria innocua, and Listeria welshimeri can ferment the sugar, producing acid which can be identified using an appropriate pH indicator. It has been used to synthesize a series of tri- and tetrahydroxylated seven-membered imino sugars in a study that worked towards a stable Noeuromycin (glycosyl cation mimic that strongly inhibits glycosidases) analog with a D-manno configuration. It has also been used in a study to investigate the primary mannose-binding site of Pradimicin A. (antifungal agent)
애플리케이션
Methyl α-D-mannopyranoside can be used to identify different species of Listeria based on their ability to ferment the sugar.
기타 정보
Unwanted binding of avidin to endogenous lectins
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
404.1 °F
Flash Point (°C)
206.74 °C
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Prevention of nonspecific binding of avidin.
R C Duhamel et al.
Methods in enzymology, 184, 201-207 (1990-01-01)
Oliver Schwardt et al.
Bioorganic & medicinal chemistry, 19(21), 6454-6473 (2011-10-04)
Urinary tract infection (UTI) caused by uropathogenic Escherichia coli (UPEC) is one of the most prevalent infectious diseases. Particularly affected are women, who have a 40-50% risk to experience at least one symptomatic UTI episode at some time during their
Karen T Welch et al.
Bioorganic & medicinal chemistry letters, 18(24), 6573-6575 (2008-11-08)
A virtual screening approach was used to identify new glycomimetics. The National Cancer Institute Diversity Set was docked into the carbohydrate binding site of the lectin concanavalin A (ConA). The resulting poses were analyzed and 19 molecules were tested for
Su Yu et al.
BMC gastroenterology, 9, 58-58 (2009-07-25)
GP2 is the major membrane protein present in the pancreatic zymogen granule, and is cleaved and released into the pancreatic duct along with exocrine secretions. The function of GP2 is unknown. GP2's amino acid sequence is most similar to that
Rafael Maldonado-Hernández et al.
Analytical biochemistry, 610, 113887-113887 (2020-08-09)
Over the past 10 years we have been developing a multi-attribute analytical platform that allows for the preparation of milligram amounts of functional, high-pure, and stable Torpedo (muscle-type) nAChR detergent complexes for crystallization purpose. In the present work, we have
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.