추천 제품
Grade
analytical standard
Quality Level
제품 라인
VETRANAL®
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
mp
159-161 °C (lit.)
항생제 활성 스펙트럼
Gram-negative bacteria
parasites
응용 분야
clinical testing
형식
neat
동작 모드
DNA synthesis | interferes
저장 온도
2-8°C
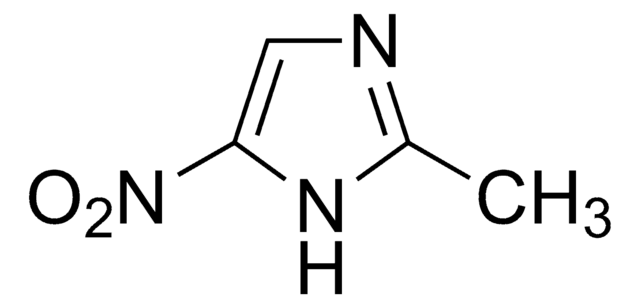
SMILES string
CC1=NC=C([N+]([O-])=O)N1CCO
InChI
1S/C6H9N3O3/c1-5-7-4-6(9(11)12)8(5)2-3-10/h4,10H,2-3H2,1H3
InChI key
VAOCPAMSLUNLGC-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chemical structure: imidazole
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
추천 제품
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
법적 정보
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 1B - Muta. 1B - STOT RE 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Sonja Löfmark et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 50 Suppl 1, S16-S23 (2010-01-14)
Metronidazole has been used for the treatment of infections for >45 years and is still successfully used for the treatment of trichomoniasis, amoebiasis, and giardiasis. Anaerobic bacterial infections caused by Bacteroides species, fusobacteria, and clostridia respond favorably to metronidazole therapy.
Konstantinos Z Vardakas et al.
International journal of antimicrobial agents, 40(1), 1-8 (2012-03-09)
The objective of this review was to evaluate the frequency of treatment failure and recurrence of Clostridium difficile infection (CDI) following treatment with vancomycin or metronidazole in recently performed studies (last 10 years). Searches in PubMed and Scopus were performed
T H I Brummer et al.
BJOG : an international journal of obstetrics and gynaecology, 120(10), 1269-1276 (2013-06-22)
To evaluate cefuroxime and metronidazole antibiotic prophylaxis. Observational nonrandomised 1-year prospective cohort study. Fifty-three hospitals in Finland. A total of 5279 women undergoing hysterectomy for benign indications, with cefuroxime given to 4301 and metronidazole given to 2855. Excluding other antibiotics
Christina M Surawicz et al.
The American journal of gastroenterology, 108(4), 478-498 (2013-02-27)
Clostridium difficile infection (CDI) is a leading cause of hospital-associated gastrointestinal illness and places a high burden on our health-care system. Patients with CDI typically have extended lengths-of-stay in hospitals, and CDI is a frequent cause of large hospital outbreaks
Amber Howerton et al.
The Journal of infectious diseases, 207(10), 1498-1504 (2013-02-20)
Clostridium difficile infection (CDI) is a leading cause of antibiotic-associated diarrhea. The infective form of C. difficile is the spore, but the vegetative bacterium causes the disease. Because C. difficile spore germination is required for symptomatic infection, antigermination approaches could
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.