1.06187
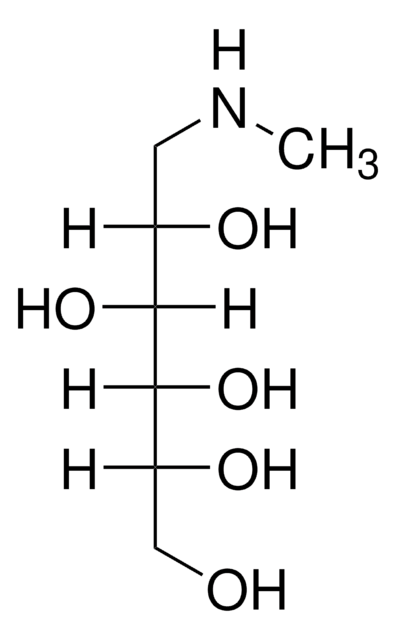
Meglumine
EMPROVE® EXPERT, Ph. Eur., ChP, JP, USP
동의어(들):
N-Methyl-D-glucamine, 1-Deoxy-1-methylaminosorbitol, 1-Deoxy-1-methylaminosorbitol, 1-Deoxy-1-(methylamino)-D-glucitol, Meglumine
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C7H17NO5
CAS Number:
Molecular Weight:
195.21
Beilstein:
385906
MDL number:
UNSPSC 코드:
12352104
EC 인덱스 번호:
228-506-9
추천 제품
Agency
ChP
JP
Ph. Eur.
USP
Quality Level
제품 라인
EMPROVE® EXPERT
양식
solid
autoignition temp.
~662 °F
pH
11 (20 °C, 10 g/L in H2O)
bp
210 °C/1013 hPa
mp
129-131.5 °C (lit.)
응용 분야
liquid formulation
pharmaceutical
solid formulation
solubility enhancement
저장 온도
no temp limit
SMILES string
CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO
InChI
1S/C7H17NO5/c1-8-2-4(10)6(12)7(13)5(11)3-9/h4-13H,2-3H2,1H3/t4-,5+,6+,7+/m0/s1
InChI key
MBBZMMPHUWSWHV-BDVNFPICSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
When working on a pharmaceutical formulation, there are specific considerations to make and challenging hurdles to take before you can successfully launch your final drug product - formulation stability, release kinetics and bioavailability limitations just to name a few. With our application know-how and regulatory expertise, we support you in every step of development, scale-up, and production.
As part of our Emprove® Program, our raw materials are offered with extensive documentation facilitating compliance of your pharma and biopharma product, full supply chain transparency and risk mitigation. Our SAFC® portfolio of high-quality products for biopharmaceutical and pharmaceutical formulation and production withstands strict quality control procedures and is produced according to applicable cGMP guidelines.
As part of our Emprove® Program, our raw materials are offered with extensive documentation facilitating compliance of your pharma and biopharma product, full supply chain transparency and risk mitigation. Our SAFC® portfolio of high-quality products for biopharmaceutical and pharmaceutical formulation and production withstands strict quality control procedures and is produced according to applicable cGMP guidelines.
애플리케이션
Meglumine Emprove® Essential is a robust pharmaceutical excipient that can provide stability, solubility enhancement and pH modification in solid and liquid formulations.
특징 및 장점
- Solubility and bioavailability enhancement of APIs
- Excellent counterion performance, a viable alternative to sodium
- API grade: manufactured under the cGMP ICH Q7 guideline for APIs
- Sole manufacturing location for API-grade meglumine in Europe
- Multi-compendial product, complying with all major pharmacopeias, including ChP
- Meglumine Emprove® API has also been successfully co-reviewed in China
법적 정보
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFC is a registered trademark of Merck KGaA, Darmstadt, Germany
또한 이 제품과 함께 일반적으로 구입
애플리케이션
제품 번호
설명
가격
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.