추천 제품
분석
>99% (TLC)
양식
powder
포장
pkg of 1 × 5 mg (700140P-5mg)
제조업체/상표
Avanti Research™ - A Croda Brand 700140P
배송 상태
dry ice
저장 온도
−20°C
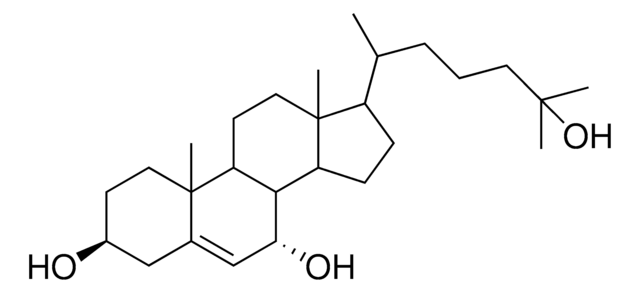
SMILES string
O[C@]21[C@@]([C@@H]3[C@H]([C@H]4[C@@]([C@H](CC4)[C@@H](CCCC(C)C)C)(CC3)C)CC2=O)(CC[C@@H](C1)O)C
InChI
1S/C27H46O3/c1-17(2)7-6-8-18(3)21-9-10-22-20-15-24(29)27(30)16-19(28)11-14-26(27,5)23(20)12-13-25(21,22)4/h17-23,28,30H,6-16H2,1-5H3/t18-,19+,20+,21-,22+,23+,25-,26-,27+/m1/s1
InChI key
SJZZRXMQSAXCFD-ZCBMJONGSA-N
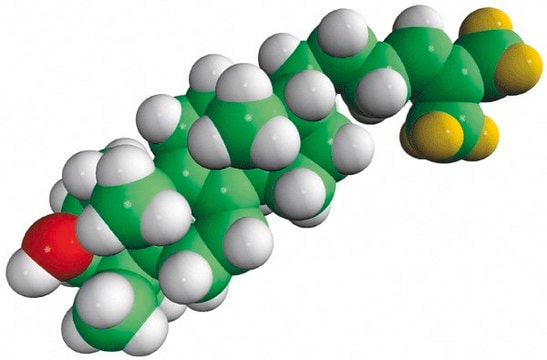
일반 설명
6-keto-5α-hydroxycholesterol, also known as 6-oxo-cholestan-3β,5α-diol (OCDO), is synthesized from the oxidation of 5β, 6β-epoxycholesterol in the presence of enzyme cholesterol epoxide hydrolase. It is also synthesized from cholestane-3β,5α,6β-triol by the enzyme 11β-hydroxysteroid dehydrogenase type II . The enzyme 11β-hydroxysteroid dehydrogenase type I catalyzes the reduction of OCDO to cholestane-3β,5α,6β-triol.
생화학적/생리학적 작용
6-keto-5α-hydroxycholesterol favors tumor progression. cholestan-6-oxo-3β,5α-diolelicits cytotoxicity towards human bronchial 16-HBE cells and plays a key role in mediating cell necrosis post ozone exposure. It is an oncometabolite, which promotes breast cancer progression and also blocks chemotaxis mediated by polymorphonuclear leukocytes.
포장
5 mL Amber Glass Screw Cap Vial (700140P-5mg)
법적 정보
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
가장 최신 버전 중 하나를 선택하세요:
Marc Poirot et al.
Biochimie, 153, 139-149 (2018-04-15)
Oxygenation products of cholesterol, named oxysterols, were suspected since the 20th century to be involved in carcinogenesis. Among the family of oxysterol molecules, cholesterol-5,6-epoxides (5,6-EC) retained the attention of scientists because they contain a putative alkylating epoxide group. However, studies
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.