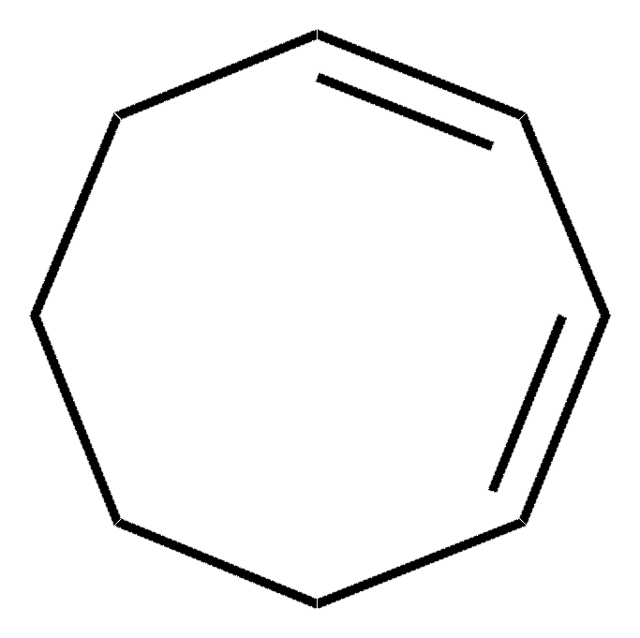
추천 제품
vapor pressure
25.8 mmHg ( 37.7 °C)
6.8 mmHg ( 25 °C)
Quality Level
분석
99%
양식
liquid
autoignition temp.
431 °F
포함
50-150 ppm 4-tert-Butylcatechol as stabilizer
50-150 ppm 4-tert-Butylcatechol
refractive index
n20/D 1.493 (lit.)
bp
149-150 °C (lit.)
mp
−69 °C (lit.)
density
0.882 g/mL at 25 °C (lit.)
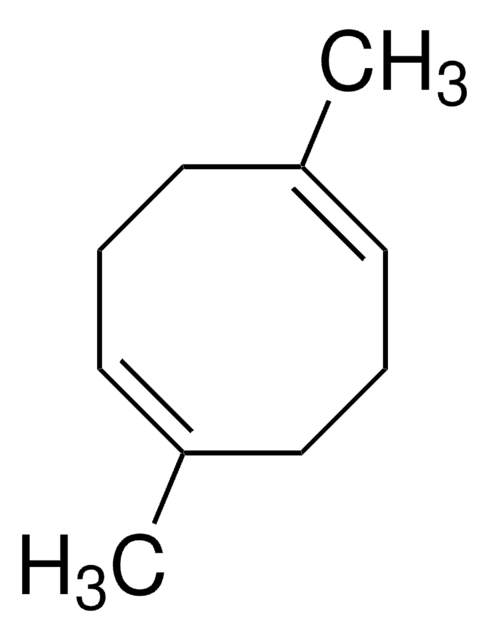
SMILES string
C1CC=CCCC=C1
InChI
1S/C8H12/c1-2-4-6-8-7-5-3-1/h1-2,7-8H,3-6H2/b2-1-,8-7-
InChI key
VYXHVRARDIDEHS-QGTKBVGQSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Bis(Aluminyl)Magnesium: A Source of Nucleophilic or Radical Aluminium-Centred Reactivity.: Investigates 1,5-Cyclooctadiene′s ability to stabilize reactive intermediates in metal complexes, which is crucial for developing new pharmaceutical agents and enhancing ligand efficiency in transition metal catalysis (Griffin et al., 2024).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
100.4 °F - closed cup
Flash Point (°C)
38 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Eleonora Cavallari et al.
The journal of physical chemistry. B, 119(31), 10035-10041 (2015-07-15)
Hyperpolarization of (13)C carboxylate signals of metabolically relevant molecules, such as acetate and pyruvate, was recently obtained by means of ParaHydrogen Induced Polarization by Side Arm Hydrogenation (PHIP-SAH). This method relies on functionalization of the carboxylic acid with an unsaturated
Adrian Tlahuext-Aca et al.
Dalton transactions (Cambridge, England : 2003), 43(42), 15997-16005 (2014-09-19)
Ni(0)-catalyzed dehydrogenation of benzylic-type imines was performed to yield asymmetrical tetra-substituted imidazoles and 2-imidazolines. This was achieved with a single operational step while maintaining good selectivity and atom economy. The catalytic system shows low to moderate tolerance for fluoro-, trifluoromethyl-
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.