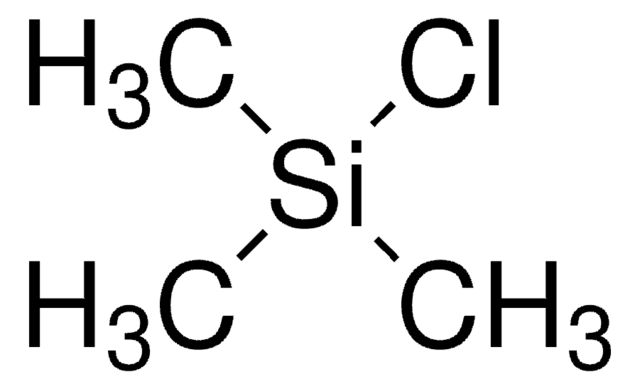95541
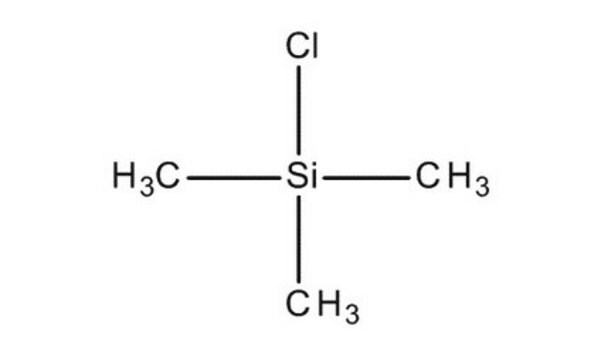
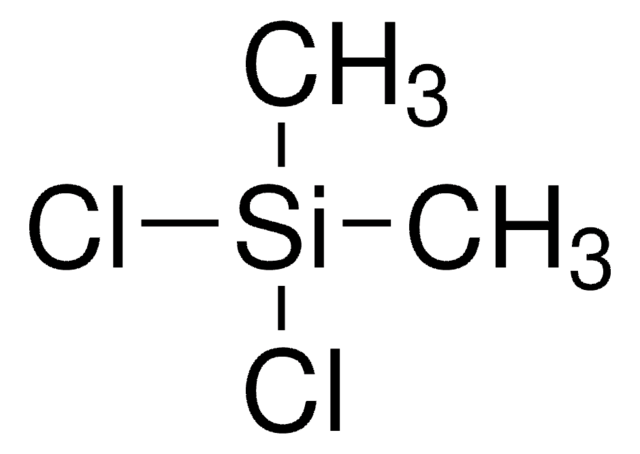
Chlorotrimethylsilane
Wacker Chemie AG, ≥99.0% (GC)
동의어(들):
Silane M3, TMCS, Trimethylchlorosilane, Trimethylsilyl chloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
(CH3)3SiCl
CAS Number:
Molecular Weight:
108.64
Beilstein:
1209232
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor density
3.7 (vs air)
Quality Level
vapor pressure
100 mmHg ( 25 °C)
분석
≥99.0% (GC)
양식
liquid
autoignition temp.
752 °F
expl. lim.
6.4 %
제조업체/상표
Wacker Chemie AG
refractive index
n20/D 1.387 (lit.)
bp
57 °C (lit.)
mp
−40 °C (lit.)
density
0.856 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(C)Cl
InChI
1S/C3H9ClSi/c1-5(2,3)4/h1-3H3
InChI key
IJOOHPMOJXWVHK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chlorotrimethylsilane is a chloro-organosilane compound mainly used for silylation reactions.
애플리케이션
Possible usages of chlorotrimethylsilane:
It is also used in Fisher glycosylation.
- Chloromethylation of polysulfones (PSUs).
- Activation of lithium hydride to convert it into a hydride source for the reductive silylation of carbonyl compounds.
- Chlorotrimethylsilane/lithium bromide forms an effective reagent for the conversion of alcohols to bromides.
- Chlorotrimethylsilane is a non-toxic alternative to mercuric chloride in activating in samarium-promoted cyclopropanation of both allylic and α-allenic alcohols.
It is also used in Fisher glycosylation.
기타 정보
Silane M3
prices for bulk quantities on request
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
-18.4 °F - closed cup
Flash Point (°C)
-28 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles
이미 열람한 고객
Science of Synthesis: Houben-Weyl Methods of Molecular Transformations, 4, 1060-1060 (2014)
A practical method for activation of commercial lithium hydride: reductive silylation of carbonyl compounds with lithium hydride and chlorotrimethylsilane.
Ohkuma T, et al.
The Journal of Organic Chemistry, 59(1), 217-221 (1994)
Modification of polysulfones by click chemistry: amphiphilic graft copolymers and their protein adsorption and cell adhesion properties.
Yilmaz G, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 49(1), 110-117 (2011)
Chlorotrimethylsilane as an Activating Reagent in the Samarium-Promoted Cyclopropanation of Allylic and ?-Allenic Alcohols.
Lautens M
The Journal of Organic Chemistry, 61(6), 2210-2214 (1996)
Ainara Sistiaga et al.
PloS one, 9(6), e101045-e101045 (2014-06-26)
Neanderthal dietary reconstructions have, to date, been based on indirect evidence and may underestimate the significance of plants as a food source. While zooarchaeological and stable isotope data have conveyed an image of Neanderthals as largely carnivorous, studies on dental
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.