912654
N-(3,5-Bis(trifluoromethyl)phenyl)-2-chloroacetamide
≥95%
동의어(들):
N-Chloroacetyl-3,5-bis(trifluoromethyl)aniline, Electrophilic scout fragment, KB03, Scout fragment for targetable cysteine
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C10H6ClF6NO
CAS Number:
Molecular Weight:
305.60
MDL number:
UNSPSC 코드:
12352200
추천 제품
분석
≥95%
양식
powder
InChI
1S/C10H6ClF6NO/c11-4-8(19)18-7-2-5(9(12,13)14)1-6(3-7)10(15,16)17/h1-3H,4H2,(H,18,19)
InChI key
LEYIUTOAQOUAFG-UHFFFAOYSA-N
애플리케이션
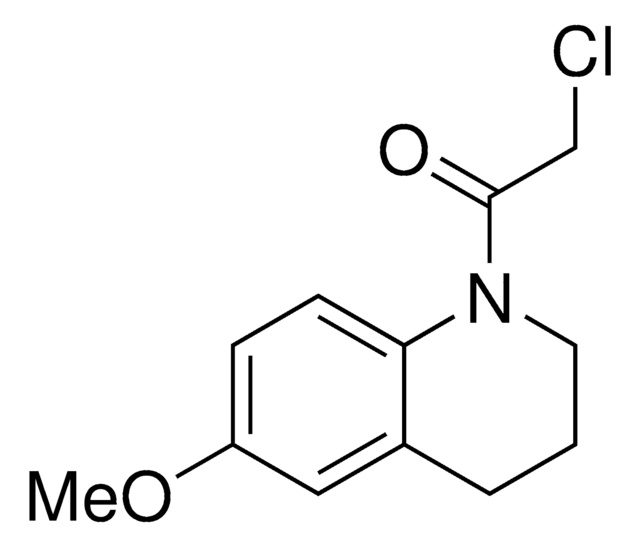
2-Chloro-1-(6-methoxy-1,2,3,4-tetrahydroquinolin-1-yl)ethan-1-one is a cysteine-reactive small-molecule fragment for chemoproteomic and ligandability studies for both traditionally druggable proteins as well as "undruggable," or difficult-to-target, proteins. This fragment electrophile, or "scout" fragment, can be used alone in fragment-based covalent ligand discovery or incorporated into bifunctional tools such as electrophilic PROTAC® molecules for targeted protein degradation as demonstrated by the Cravatt Lab for E3 ligase discovery.
기타 정보
법적 정보
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Péter Ábrányi-Balogh et al.
European journal of medicinal chemistry, 160, 94-107 (2018-10-16)
Targeted covalent inhibitors have become an integral part of a number of therapeutic protocols and are the subject of intense research. The mechanism of action of these compounds involves the formation of a covalent bond with protein nucleophiles, mostly cysteines.
De-Wei Gao et al.
Journal of the American Chemical Society, 140(26), 8069-8073 (2018-06-13)
Nucleophilic attack on carbon-based electrophiles is a central reactivity paradigm in chemistry and biology. The steric and electronic properties of the electrophile dictate its reactivity with different nucleophiles of interest, allowing the opportunity to fine-tune electrophiles for use as coupling
Keriann M Backus et al.
Nature, 534(7608), 570-574 (2016-06-17)
Small molecules are powerful tools for investigating protein function and can serve as leads for new therapeutics. Most human proteins, however, lack small-molecule ligands, and entire protein classes are considered 'undruggable'. Fragment-based ligand discovery can identify small-molecule probes for proteins
문서
Ligandability describes the propensity of a protein target to bind a small molecule with high affinity. It is a precursor to evaluating druggability, which requires more advanced translational pharmacological effects and drug-like properties in vivo.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.