856061
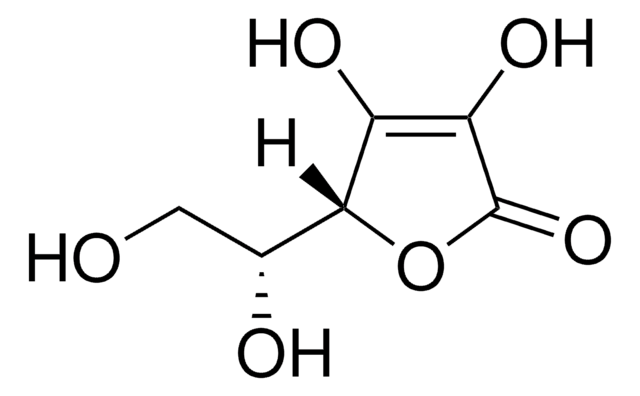
D-(−)-Isoascorbic acid
98%
동의어(들):
D-erythro-Hex-2-enoic acid γ-lactone, D-Araboascorbic acid, Erythorbic acid, Glucosaccharonic acid, NSC 8117
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C6H8O6
CAS Number:
Molecular Weight:
176.12
Beilstein:
84271
EC Number:
MDL number:
UNSPSC 코드:
12352205
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
양식
crystals
광학 활성
[α]25/D −16.8°, c = 2 in H2O
mp
169-172 °C (dec.) (lit.)
SMILES string
[H][C@@]1(OC(=O)C(O)=C1O)[C@H](O)CO
InChI
1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5-/m1/s1
InChI key
CIWBSHSKHKDKBQ-DUZGATOHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
D-(−)-Isoascorbic acid, also known as erythorbic acid, is widely utilized as a chiral building block in organic synthesis for the preparation of various chiral compounds. It is also used as a reducing agent in various organic reactions.
애플리케이션
D-(−)-Isoascorbic acid can be used as a reactant in the synthesis of various chiral compounds such as:
- enantiopure aminotriol
- (3R, 4S)-4-hydroxylasiodiplodin and D-mycinose
- enantiomerically pure stereoisomers of α,β-dihydroxy-aldehydes or acids
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Andrew C Clark et al.
Journal of agricultural and food chemistry, 58(2), 1004-1011 (2009-12-31)
The stereochemical influence of antioxidant and flavanol compounds on oxidation processes in a model wine system was studied. The diastereoisomers, ascorbic acid and erythorbic acid, were used as antioxidants in a model wine system containing either (+)-catechin or (-)-epicatechin as
Manuel Bueno et al.
Carbohydrate research, 344(15), 2100-2104 (2009-07-17)
l-Ascorbic and d-isoascorbic acids have been used as the starting materials for the preparation of (3R,4'S)-3-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPTA), (3R and S, 4'S,6R)-3-methyl-6-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPTP) and (3R,4'R)-3-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPEA), three novel 1,4-dioxane-2,5-dione-type monomers. Ring-opening homopolymerisation and copolymerisation of the IPTA monomer, derived from l-ascorbic
Spyros Drivelos et al.
Analytical and bioanalytical chemistry, 397(6), 2199-2210 (2010-04-16)
A new hydrophilic interaction liquid chromatographic (HILIC) method for the simultaneous determination of isoascorbic (IAA) and ascorbic acid (AA) was developed. The separation of IAA and AA was studied in various HILIC stationary phases and the influence of the composition
Daiki Kyotani et al.
Bioscience, biotechnology, and biochemistry, 73(4), 954-956 (2009-04-09)
We found that a strain of Penicillium sp. effectively converted L-ascorbic acid to a five-carbon analog, which was identified as L-erythroascorbic acid based on spectroscopic analysis. The conversion was achieved by growing culture or washed mycelia, with a yield of
Alberto Baroja-Mazo et al.
Fungal genetics and biology : FG & B, 42(5), 390-402 (2005-04-06)
D-Erythroascorbate and D-erythroascorbate glucoside have been identified in the Zygomycete fungus Phycomyces blakesleeanus. Ascomycete and Basidiomycete fungi also synthesise D-erythroascorbate instead of l-ascorbate, suggesting that D-erythroascorbate synthesis evolved in the common ancestor of the fungi. Both compounds accumulate in P.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.