774138
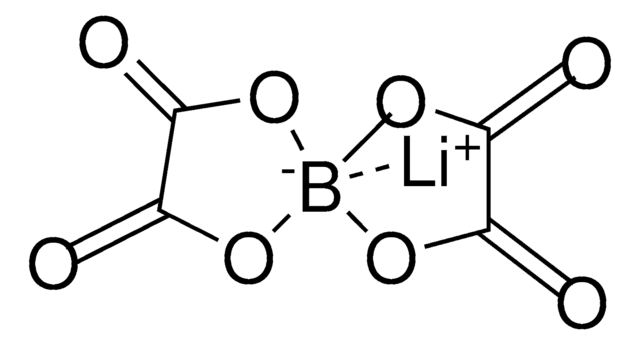
Lithium difluoro(oxalato)borate
동의어(들):
LIDFOB, LIF2OB, LIFOB, LIODFB, Lithium difluoro(ethanedioato)borate, Lithium oxalatodigluoroborate
About This Item
추천 제품
양식
powder
Quality Level
환경친화적 대안 제품 특성
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
265-271 °C
응용 분야
battery manufacturing
환경친화적 대안 카테고리
, Enabling
SMILES string
F[B-]1(OC(C(O1)=O)=O)F.[Li+]
InChI
1S/C2BF2O4.Li/c4-3(5)8-1(6)2(7)9-3;/q-1;+1
InChI key
MEDDCIKGDMDORY-UHFFFAOYSA-N
일반 설명
애플리케이션
특징 및 장점
✔ Increases battery life
✔ Stabilizes SEI layer
✔ Suitable for fast charging and low temperatures
관련 제품
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
문서
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
Due to the adverse impact of the continued use of fossil fuels on the earth’s environment and climate, researchers have been asked to develop new approaches for producing power using renewable sources like wind and solar energy
관련 콘텐츠
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.