모든 사진(1)
About This Item
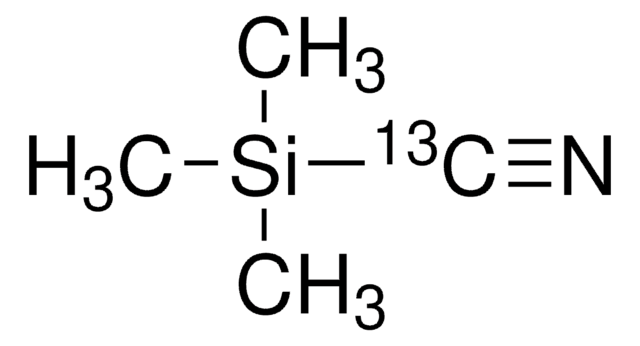
Linear Formula:
(CH3)3SiCN
CAS Number:
Molecular Weight:
99.21
Beilstein:
1737612
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
grade
technical
Quality Level
분석
≥95% (GC)
양식
liquid
refractive index
n20/D 1.392 (lit.)
bp
114-117 °C (lit.)
mp
8-11 °C (lit.)
density
0.793 g/mL at 20 °C (lit.)
SMILES string
C[Si](C)(C)C#N
InChI
1S/C4H9NSi/c1-6(2,3)4-5/h1-3H3
InChI key
LEIMLDGFXIOXMT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Trimethylsilyl cyanide (TMSCN) can be used as a reagent in the:
- Cyanosilylation of carbonyl compounds using various catalysts.
- Synthesis of α-aminonitriles by one-pot, three-component Strecker reaction of ketones with various amines using Brønsted acid catalyst.
- Cyanation of aryl halides using palladium-complex as a catalyst.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Liq. 2
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
33.8 °F - closed cup
Flash Point (°C)
1 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Indium tribromide: a highly effective catalyst for the addition of trimethylsilyl cyanide to ?-hetero-substituted ketones
Bandini M, et al.
Tetrahedron Letters, 42(16), 3041-3043 (2001)
Asymmetric addition of trimethylsilyl cyanide to ketones catalyzed by Al (salen)/triphenylphosphine oxide
Kim SS and Kwak Ju M
Tetrahedron, 62(1), 49-53 (2006)
A convenient and efficient procedure for the palladium-catalyzed cyanation of aryl halides using trimethylsilylcyanide
Sundermeier M, et al.
Journal of Organometallic Chemistry, 684(1-2), 50-55 (2003)
Mikhail D Kosobokov et al.
The Journal of organic chemistry, 77(13), 5850-5855 (2012-06-20)
A new silicon reagent, difluoro(trimethylsilyl)acetonitrile, was prepared by insertion of difluorocarbene into silyl cyanide. The obtained silane served as a good cyanodifluoromethylating reagent toward aldehydes, N-tosylimines, N-alkylimines, and enamines under basic or acidic conditions.
Enantioselective synthesis of tertiary α-hydroxy phosphonates catalyzed by carbohydrate/cinchona alkaloid thiourea organocatalysts.
Shasha Kong et al.
Angewandte Chemie (International ed. in English), 51(35), 8864-8867 (2012-08-01)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.