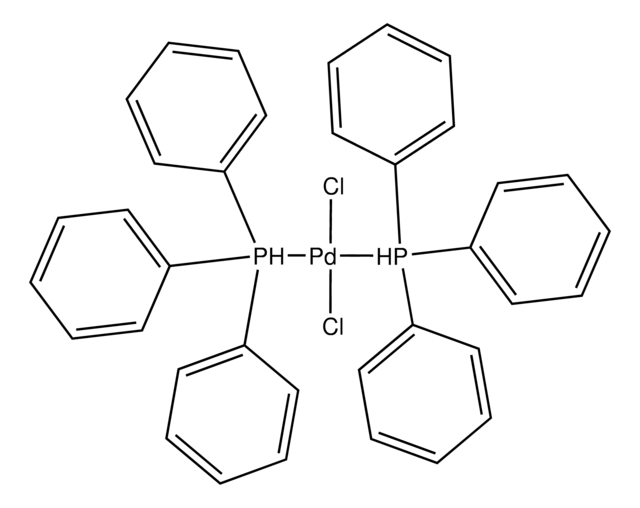
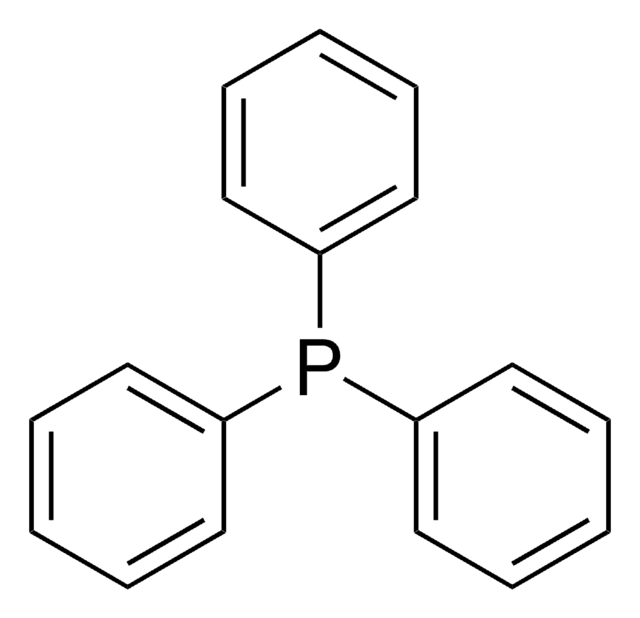
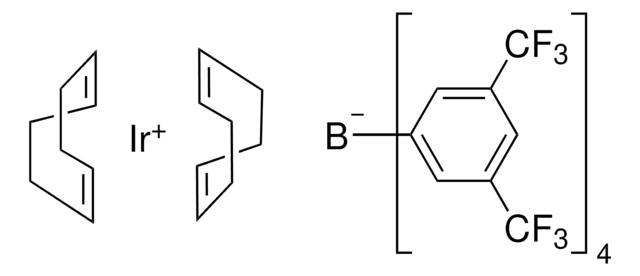
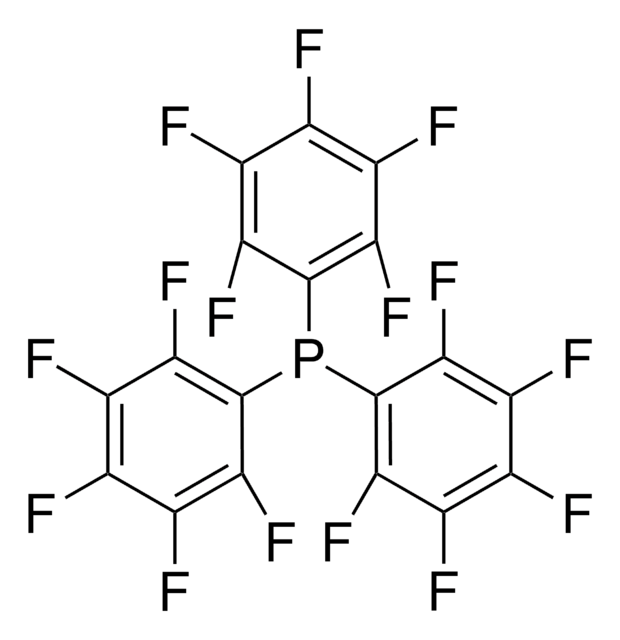
추천 제품
양식
crystals
Quality Level
반응 적합성
core: iridium
reagent type: catalyst
reaction type: C-H Activation
mp
154-179 °C (D)
저장 온도
−20°C
SMILES string
C[O+]1[Ir-]2[O+](C)[Ir-]12.C3CC=CCCC=C3.C4CC=CCCC=C4
InChI
1S/2C8H12.2CH3O.2Ir/c2*1-2-4-6-8-7-5-3-1;2*1-2;;/h2*1-2,7-8H,3-6H2;2*1H3;;/q;;2*+1;2*-1/b2*2-1-,8-7-;;;;
InChI key
BGWIAAATAAWGOI-MIXQCLKLSA-N
애플리케이션
A powerful C-H activation catalyst to prepare phenols from arenes
Catalyst for:
- Preparation of heteroaryl fused indole ring systems as inhibitors of HCV NS5B polymerase
- Borylation/Suzuki-Miyaura coupling
- Metalation-Suzuki cross-coupling procedure for the synthesis of biaryls and heterobiaryls
- Tetraborylation reactions
- Highly regio- and enantioselective asymmetric hydroboration
- Ortho-silylation of aryl ketone, benzaldehyde, and benzyl alcohol derivatives via C-H activation
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
A general strategy for the perfluoroalkylation of arenes and arylbromides by using arylboronate esters and [(phen) CuRF].
Litvinas N, et al.
Angewandte Chemie (International Edition in English), 51(2), 536-539 (2012)
Ir?Catalyzed Borylation of C H Bonds in N?Containing Heterocycles: Regioselectivity in the Synthesis of Heteroaryl Boronate Esters.
Mkhalid I, et al.
Angewandte Chemie (International Edition in English), 45(3), 489-491 (2006)
Direct C?H borylation and C?H arylation of pyrrolo [2, 3-d] pyrimidines: synthesis of 6, 8-disubstituted 7-deazapurines.
Klecka M, et al.
Organic & Biomolecular Chemistry, 7(5), 866-868 (2009)
Catalytic functionalization of unactivated primary C?H bonds directed by an alcohol.
Simmons E, et al.
Nature, 483(7387), 70-70 (2012)
Robert E Maleczka et al.
Journal of the American Chemical Society, 125(26), 7792-7793 (2003-06-26)
An efficient one-pot C-H activation/borylation/oxidation protocol for the preparation of phenols is described. This method is particularly attractive for the generation of meta-substituted phenols bearing ortho-/para-directing groups, as such substrates are difficult to access by other phenol syntheses.
문서
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)